De Gruyter STEM
ISBN 9783110782042
e-ISBN (PDF) 9783110782134
e-ISBN (EPUB) 9783110782257
Bibliographic information published by the Deutsche Nationalbibliothek
The Deutsche Nationalbibliothek lists this publication in the Deutsche Nationalbibliografie; detailed bibliographic data are available on the Internet at http://dnb.dnb.de.
2022 Walter de Gruyter GmbH, Berlin/Boston
Flash Point
Flash point (FP) of an organic liquid compound is the lowest temperature at which a liquid can form an ignitable mixture in air near the surface of the liquid where its measurement requires an ignition source. The American Society for Testing and Materials (ASTM) defines FP under specified testing conditions and 101.3 kPa pressure []. The FP data are widely used to evaluate the fire and explosion hazards of liquids. They have great practical significance in the handling and transporting of such chemicals in bulk quantities. Above the FP temperature, a liquid can produce enough vapor to form a flammable mixture with air. Organic liquids with lower FP can be ignited more easily because the FP of a volatile organic compound is the lowest temperature at which vapors of a fluid will ignite. Fire point is the temperature at which the vapor continues to burn after being ignited. When the ignition source is removed at the FP, which is different from the fire point, the vapor may cease to burn. Since FP and the fire point do not depend on the temperature of the ignition source, a certain concentration of vapor for each flammable liquid in the air is necessary to sustain combustion. Thus, when an ignition source of sufficient strength is applied, the FP of an organic flammable liquid is the lowest temperature at which there will be enough flammable vapor to ignite.
The FP of a pure flammable organic compound or mixture is an important parameter in hazard classification of flammable liquids. Since the FP is an approximation of the lower temperature limit, the temperature at which a chemical compound evolves enough vapors to support combustion, it is essential to be updated for safe handling, transportation, and storage of many substances [].
1.1 Measurement of the FP
There are five main apparatuses used internationally for measurement of the FP that are given in ]. Experimental determinations of the FP are described in various sources, which differ in their scope and experimental conditions as:
The ASTM D56 [) is used for materials with viscosities lower than 5.5 mm2/s and the FP below 93 C.
The ASTM D93 [) is used for materials with the FP between 40 C and 360 C, such as distillate fuels, lubricating oils, fuel oils, mixtures of petroleum liquids with solids, and biodiesel fuels.
The ASTM D92 [) is used for liquids with high viscosity and the FP between 79 C and 400 C, such as petroleum products except for fuel oils.
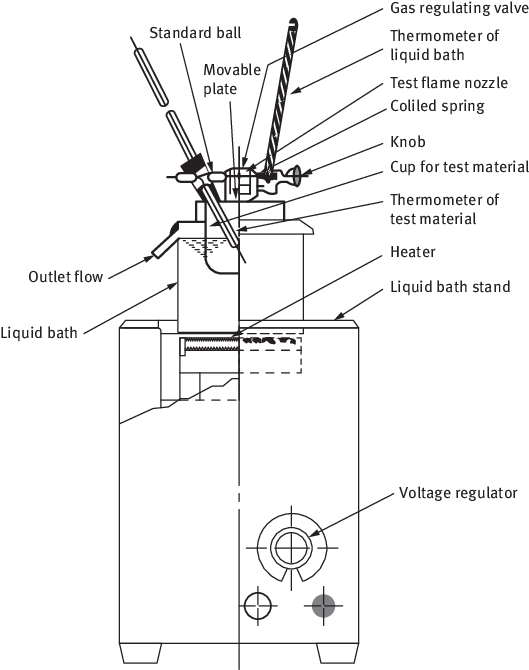
Figure 1.1: Tag closed cup method for measuring the FP.

Figure 1.2: Pensky-Martens closed cup tester.

Figure 1.3: Cleveland open cup method.
Table 1.1: Five main FP devices currently used.
| Device | Temperature uniformity | Sample volume (mL) | Main use |
|---|
| Tagliabue (Tag) | Liquid Bath | 50 | Less viscous compounds |
| Pensky-Martens | Stirred; Metal shell | 75 | Viscous compounds |
| Cleveland | Metal plate across cup base | 70 | Open cup tests |
| Small-Scale (Setaflash) | Preheated to fixed temperature | 2 or 4 | Small-scale and flash/no-flash tests |
| Abel | Water bath and air gap | 79 | European tests |
Table 1.2: ASTM standardized method for measuring the FP.
| Method | Device | Open/Closed | Heating rate Cmin1 | Range (C) |
|---|
| D 56 | Tag | Closed | 13 | <93 |
| D 92 | Cleveland | Open | 56 | 79400 |
| D 93 | Pensky-Martens | Closed | 45 | 40370 |
| D 1310 | Tag | Open | 1 | 18 to 165 |
| D 3278 | Small-scale | Closed | Fixed temperature | 0110 |
| D 3828 | Small-scale | Closed | Fixed temperature | 20 to 300 |
| D 3941 | Tag or Pensky-Martens | Closed | Fixed temperature | 0110 |
| D 3941 | Tag or Pensky-Martens | Closed | 0.5 | <93 or 40370 |
].
Different factors can affect the measured FP values, which include []:
Sample size as well as its viscosity and homogeneity (in mixtures)
The type, position, and dimension of the ignition source
Temperature rise rate
Stirring of liquid
Mixing vapor phase above pool
Drafts
Ambient pressure
Operator bias
Fuel container condition (open or closed)
Thus, the reported experimental values of FP in literature sometimes differ tens of degrees [].
1.2 Predictive methods of the FP for pure compounds
Measurement of the FP temperature is costly and may contain high experimental uncertainties []. Thus, reliable prediction of the FP values for different classes of organic compounds is desirable.
The lack of the measured data of the FP for most newly introduced compounds in the industry has driven the development of many FP predictive models, which can be generally divided into three categories: empirical, group contribution, and quantitative structure-property relationship (QSPR) models. There are several reviews of these methods [].
1.2.1 Empirical models
Empirical models include correlations, which predict the FP by other physical properties that are more accessible or can be measured more easily, e.g., normal boiling point ] presented a simple empirical equation for estimation of the FP of pure organic liquids. Their correlation is based on NBP, the standard enthalpy of vaporization at 298.15 K[vapH(298.15K)] of the compound, and the number of carbon atoms ( nC ) in the molecule. The bounds for this correlation are 100FP(C)+200;250NBPK650;20vapH(298.15K)/(kJmol1)110; and 1nc21 . The accuracy of these empirical correlations is directly dependent on the accuracy of the measured physical properties and the predictive methods, which were used for their estimations. If one of the aforementioned properties such as the NBP is not available, it is not possible to estimate the FP property. This is the main drawback of empirical correlations.
Among different empirical methods, the procedures based on the NBP are the most widely used methods because the NBP is the most readily available thermophysical properties. It should be mentioned that the value of the FP of a substance is related directly to the NBP and inversely to the vapor pressure at a given temperature. Flammable liquids with high vapor pressures, as a general rule, at normal temperatures commonly exhibit low boiling points and low FP [].















