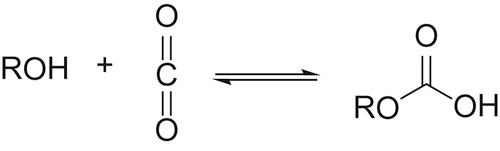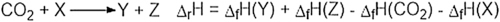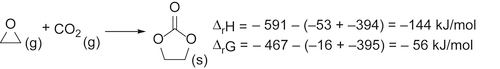Peter Styring - Carbon Dioxide Utilisation: Closing the Carbon Cycle
Here you can read online Peter Styring - Carbon Dioxide Utilisation: Closing the Carbon Cycle full text of the book (entire story) in english for free. Download pdf and epub, get meaning, cover and reviews about this ebook. year: 2014, publisher: Elsevier, genre: Children. Description of the work, (preface) as well as reviews are available. Best literature library LitArk.com created for fans of good reading and offers a wide selection of genres:
Romance novel
Science fiction
Adventure
Detective
Science
History
Home and family
Prose
Art
Politics
Computer
Non-fiction
Religion
Business
Children
Humor
Choose a favorite category and find really read worthwhile books. Enjoy immersion in the world of imagination, feel the emotions of the characters or learn something new for yourself, make an fascinating discovery.
- Book:Carbon Dioxide Utilisation: Closing the Carbon Cycle
- Author:
- Publisher:Elsevier
- Genre:
- Year:2014
- Rating:4 / 5
- Favourites:Add to favourites
- Your mark:
Carbon Dioxide Utilisation: Closing the Carbon Cycle: summary, description and annotation
We offer to read an annotation, description, summary or preface (depends on what the author of the book "Carbon Dioxide Utilisation: Closing the Carbon Cycle" wrote himself). If you haven't found the necessary information about the book — write in the comments, we will try to find it.
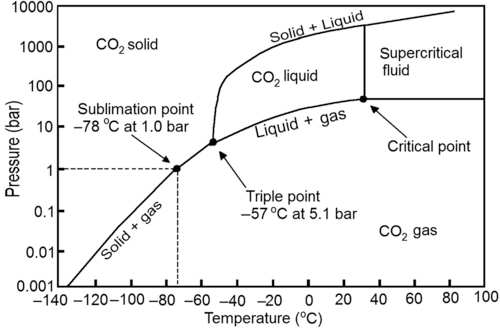
Carbon Dioxide Utilisation: Closing the Carbon Cycle explores areas of application such as conversion to fuels, mineralization, conversion to polymers, and artificial photosynthesis as well as assesses the potential industrial suitability of the various processes. After an introduction to the thermodynamics, basic reactions, and physical chemistry of carbon dioxide, the book proceeds to examine current commercial and industrial processes, and the potential for carbon dioxide as a green and sustainable resource.
While carbon dioxide is generally portrayed as a bad gas, a waste product, and a major contributor to global warming, a new branch of science is developing to convert this bad gas into useful products. This book explores the science behind converting CO2 into fuels for our cars and planes, and for use in plastics and foams for our homes and cars, pharmaceuticals, building materials, and many more useful products.
Carbon dioxide utilization is a rapidly expanding area of research that holds a potential key to sustainable, petrochemical-free chemical production and energy integration.
- Accessible and balanced between chemistry, engineering, and industrial applications
- Informed by blue-sky thinking and realistic possibilities for future technology and applications
- Encompasses supply chain sustainability and economics, processes, and energy integration
Peter Styring: author's other books
Who wrote Carbon Dioxide Utilisation: Closing the Carbon Cycle? Find out the surname, the name of the author of the book and a list of all author's works by series.











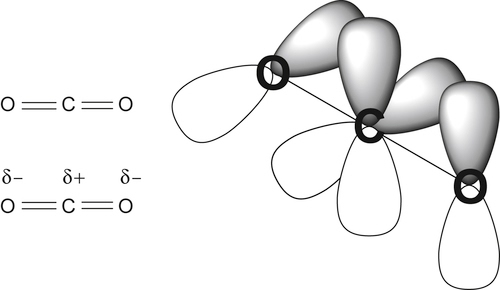
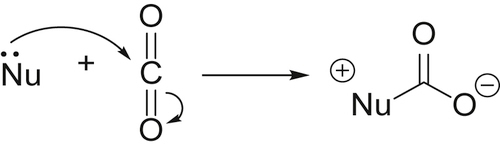
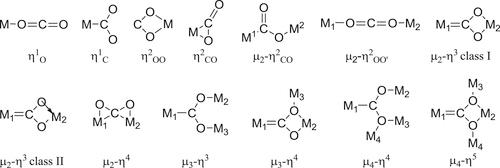
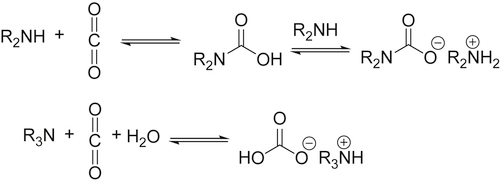
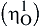 and this does not change the geometry of the carbon dioxide, but will withdraw electron density from it, thus making the carbon atom more susceptible to attack by nucleophiles. In contrast, metals with loosely held electrons may coordinate to the carbon atom of carbon dioxide (C1)
and this does not change the geometry of the carbon dioxide, but will withdraw electron density from it, thus making the carbon atom more susceptible to attack by nucleophiles. In contrast, metals with loosely held electrons may coordinate to the carbon atom of carbon dioxide (C1) 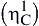 which both makes the carbon atom less electron deficient and hence less susceptible to attack by nucleophiles: this also changes the overall geometry of the CO2 unit from linear to bent.
which both makes the carbon atom less electron deficient and hence less susceptible to attack by nucleophiles: this also changes the overall geometry of the CO2 unit from linear to bent.