Addendum
As this document was going to print, the work of an American scholar, Professor Robert H. Goddard of Clark College in Worcester, Massachusetts, became known to me. The work was published in Washington in 1919 by the renowned Smithsonian Institution under the title: A Method of Reaching Extreme Altitudes and it concerns rockets.
Prof. Goddard was able to experiment with considerable resources, while I primarily had to attempt a theoretical treatment of the problem. For this reason, our works complement each other.
Goddard worked with smokeless nitrocellulose powder. He first investigated the functioning of funnel-shaped nozzles with a divergence of less than 8 and found that this greatly improved the thermal efficiency. He was able to convert up to 64% of the thermochemical energy into kinetic energy of the exhaust gases (Exp. 51), while so far, in the best common rockets, the efficiency barely exceeds 2%. Goddard further found that efficiency improves if the nozzle is made larger with a similar shape and the same ratio of the amount of powder to nozzle contents. This was naturally to be expected, because, in his experiments, the amount of powder increased by the 3rd power, while the wall surface, and therefore friction, increased only by the square of the linear quantity. After all, especially with the present state of aerodynamics, experimental confirmation of theoretical considerations is absolutely necessary.The role of friction is surprisingly small, even in small nozzles. The throat of the smaller nozzle was about cm wide; the thermal efficiency in the reported experiments was between 30 and 50%. In a larger apparatus, the nozzle throat was 1 cm wide; here the efficiency lay between 57 and 65%, proof that friction can surely only play a very minimal role here. (In my apparatus, the friction would likely be significantly higher, relatively speaking.) The nozzles that Goddard worked with were meticulously polished, and some were made of the finest steel, others of ordinary iron, but at any rate, of heavy materials. In my apparatuses, on the other hand, the nozzles are made of thin, sheet metal. This will certainly not happen without warping, and a thin layer of ice would accumulate on the nozzle wall of the hydrogen rocket, which would considerably increase the friction. But because the nozzles of my apparatuses are significantly wider, friction will not be greater.
The high level of thermal efficiency is remarkable. (The best diesel engines, for example, convert barely 40% of the thermal into mechanical energy, and steam engines do not even return 21% of the supplied heat energy as work). Goddard explains the high thermal efficiency by 1. absence of friction, 2. low heat dissipation by conduction (because the explosion takes place very rapidly) and, 3. the high combustion temperature.
Based on his sophisticated experiments, Goddard was able to draw conclusions about the exhaust velocity in airless space. He could confirm (as was to be expected according to theory) that the exhaust velocity increases with decreasing ambient air pressure and approaches a maximum value in airless space.
Goddard conducted still further experiments on apparatus materials, the shape and length of the nozzle, and so on. He reported on experiments with four different types of powder. He found that the exhaust velocity increases with increasing combustion temperature (which is also natural when the gases have about the same molecular weight). He achieved the highest velocities with the smokeless powder Infallible, produced by the Hercules Powder Company (explosion heat 1238.5 cal/g; exhaust velocity up to 2.434 km/sec), and with Du Pont pistol powder No. 3 (972.5 cal/g and 2.290 km/sec).
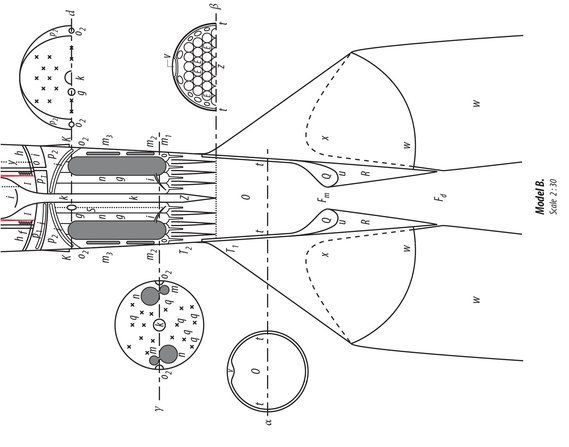
Goddard did not describe any particular apparatus. The principle he suggests is the following: The powder is packed in individual cartridges. The exhaust apparatus is relatively small and is automatically loaded and fired like a machine gun, but at a more rapid rate. The apparatus would basically serve the same purposes as my Model B. It is also (as I must frankly admit), without a doubt, more suitable than an oxygen apparatus. However, Goddards principle does not have the development opportunities, which I have discussed in the third part of my document.
Goddard also considered the use of hydrogen and oxygen, as is shown in a note (19, p. 66). However, he did not follow up on this; he only noted that the operation of the apparatus would be significantly more difficult. Perhaps he chose the explosive powder rocket because of its ease of applicability.
As for the theoretical part of Goddards work, his calculations and formulas are easier to understand than mine. But my work may, in general, bring more to this field. Goddards formulas and computations are naturally quite similar to mine.
In a beautiful way, Goddard calculated the probability of a collision between a rocket and a meteorite.
I would also like to mention that Goddard thought of sending a rocket filled with flash powder to the Moon. On impact the flash powder would ignite, thus making the impact of the apparatus visible to a viewer.
Of course, I cannot go into all the details of his paper here, but I can recommend it to all readers who are proficient in English, because the experiments were conducted meticulously, and the document is easy to understand and interestingly written.
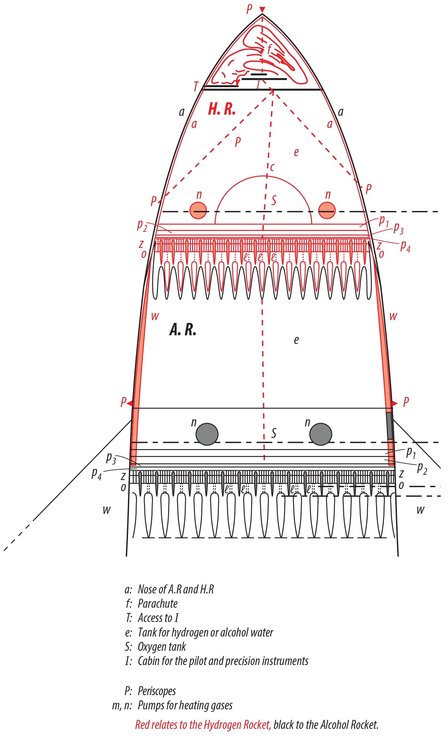
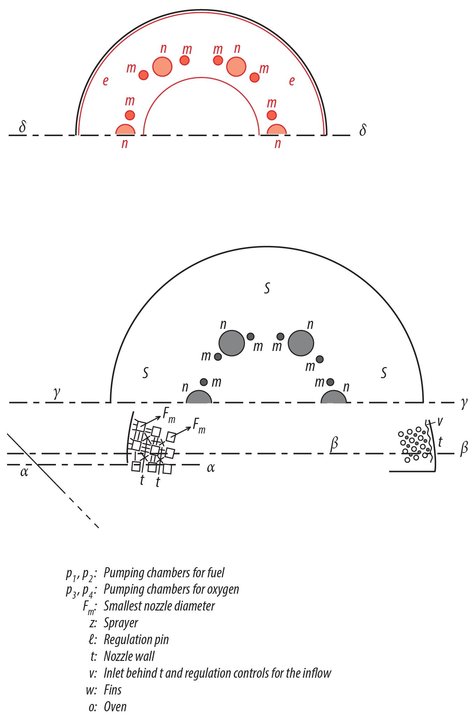
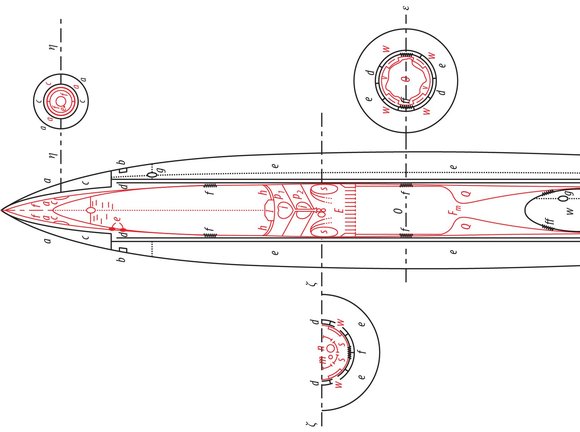
One will readily see that I proceeded independently of Goddard by comparing our publications with each other. Incidentally, I can prove through witnesses that my work goes back to 1907. My first complete plan dates from 1909. It concerned an apparatus that would be capable of carrying several people aloft. The propelling apparatus was supplied with moistened gun cotton like a machine gun. The gas would stream out laterally at the top. The form of the nozzles was still quite imperfect. They looked like the water nozzles of Pelton turbines and, like those, also had regulator pins, which worked automatically to prevent excessive Andruck . The ordnance was stored in chambers with thin, moistened walls, which would be discarded when empty. The entire thing is reminiscent of (p. 41). Despite its imperfections, an apparatus of this type would be capable of ascending. At that time, I already knew about the physical phenomena of Andruck , the formula (19) on p. 18, in addition to (1)(5), and the relationships discussed in 7) 13, 57, 17 and 18. In 1912, I drafted the first plan of an oxygen-hydrogen rocket. In 1918, I performed calculations on a small model in which the exhaust gases streamed out the bottom and the first stage was an alcohol rocket. I established formulas 611 in the summer of 1920 as I attempted to develop a complete rocket theory. I calculated the plan for Model B during the composition of this work in the winter of 1921/22 in order to demonstrate the implementation of my theory in practice, likewise quite independently of Goddard. Further details would probably be of no interest here.