Springer Science+Business Media, LLC 2018
Tracy I. George and Daniel A. Arber (eds.) Atlas of Bone Marrow Pathology Atlas of Anatomic Pathology
1. Normal Bone Marrow
The bone marrow examination is an important diagnostic procedure used for a wide variety of clinical conditions such as the diagnosis of myeloid or lymphoid neoplasms, various reactive conditions or metastatic, non-hematopoietic malignancies. Bone marrow examination is also used for confirmation or monitoring of a remission state, residual or recurrent disease state, or regeneration of bone marrow after various therapies. Bone marrow aspiration and biopsy of adequate quality are considered to represent overall bone marrow function.
A basic understanding of bone marrow structures and the correct identification of cells comprising normal bone marrow are very important in the interpretation of bone marrow pathology. The bone marrow is a well-organized structure confined in cortical bone and traversed by medullary or trabecular bone. The bone marrow has three components: hematopoietic cells, stroma/microenvironment, and medullary bone. Hematopoietic cells are embedded in a connective tissue stroma in intertrabecular spaces of medullary bone. The bone marrow is almost entirely occupied by hematopoietic cells, with the highest cellularity at birth or early infancy. The hematopoietic cells gradually decrease in the bone marrow with aging, and the bone marrow is replaced by adipose cells (fat cells). Hematopoietic cells derived from multipotent stem cells can be further differentiated into several lineage cells: erythrocytes, granulocytes, monocytes, megakaryocytes , and lymphocytes.
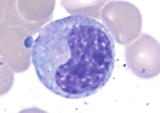
Tables list the characteristic cytologic features of erythroid cells, granulocytic cells, and megakaryocytic cells. These tables illustrate the various stages of maturation from the earliest recognizable immature cells to mature cells in the bone marrow. Erythroid precursor cells (normoblast or erythroblast) develop adjacent to macrophages and are subdivided into pronormoblasts, basophilic normoblasts, polychromatophilic normoblasts, and orthochromic normoblasts. Immature granulocytic cells develop adjacent to trabecular surfaces or arterioles and are further subdivided into blasts, promyelocytes, myelocytes, metamyelocytes, band neutrophils, and segmented neutrophils. Megakaryocytes, the largest hematopoietic cells in bone marrow, can be easily identified adjacent to sinusoids, but megakaryoblasts or immature megakaryocytes are often difficult to recognize in the bone marrow and can be readily identified in conjunction with immunohistochemistry or immunophenotype.
Table 1.1
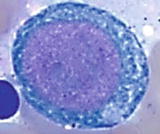
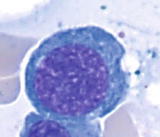
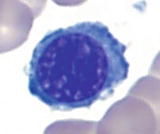
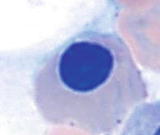
Maturation of erythroid cells in bone marrow
Cell type | Characteristic morphology | Description |
|---|
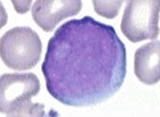
Pronormoblast (proerythroblast) | | The most immature and largest cells in erythroid lineage (1224 m), relatively high nuclear to cytoplasmic (N/C) ratio (78:1), round to slightly oval nucleus, finely reticulated chromatin, prominent nucleoli ( 1), and agranular basophilic cytoplasm |
Basophilic normoblast | | Smaller cells (1017 m) than pronormoblast, round nucleus, high N/C ratio (6:1), open to slightly condensed chromatin, distinct parachromatin, rarely visible or absent nucleoli in later stage, and deep basophilic cytoplasm |
Polychromatophilic normoblast | | Smaller cells (1015 m) and lower N/C ratio (4:1) than basophilic normoblasts, round nucleus with condensed chromatin, often cartwheel appearance, visible perinuclear halo, no nucleoli, and blue-gray to pink-gray cytoplasm |
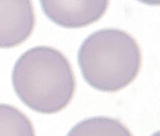
Orthochromic normoblast | | More mature and smaller cells (812 m) than polychromatophilic normoblast, abundant cytoplasm (N/C ratio 1:2) with pink-orange and minimally basophilic color similar to erythrocytes, round nucleus, and densely condensed or pyknotic chromatin |
Erythrocyte | | The most mature cells (78.5 m), pink-orange to salmon color, and no nucleus |
Table 1.2
Maturation of granulocytic cells in the bone marrow
Cell type | Characteristic morphology | Description |
|---|
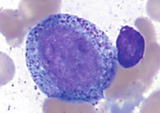
Myeloblast | | The most immature granulocytic cells (1520 m), with high N/C ratio (47:1), round to oval nucleus, fine to reticular chromatin with distinct nucleoli (15), and moderately basophilic cytoplasm with absent or minimal azurophilic granules |
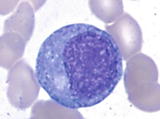
Promyelocyte | | Slightly larger cells (1424 m) than myeloblasts, with high N/C ratio (35:1), eccentric round to oval nucleus, slightly coarse or finely reticular chromatin, distinct nucleoli (13), basophilic cytoplasm with paranuclear hof and prominent azurophilic (primary) granules, which may overlie the nucleus |
Myelocyte | | Slightly smaller cells (1018 m) than blasts, with more abundant cytoplasm (N/C ratio 12:1), eccentric round to oval nucleus, more condensed chromatin, no nucleoli, bluish to pink cytoplasm with paranuclear hof, abundant lilac (secondary) granules, and scattered few azurophilic (primary) granules |
Metamyelocyte | | Size similar to or slightly smaller (1018 m) than myelocytes, with abundant cytoplasm (N/C ratio 11.5:1), indented or kidney-shaped nucleus (indentation less than half the width of the nuclear margin), condensed chromatin, no nucleoli, pinkish cytoplasm with many secondary granules and rare primary granules |
Band neutrophil |