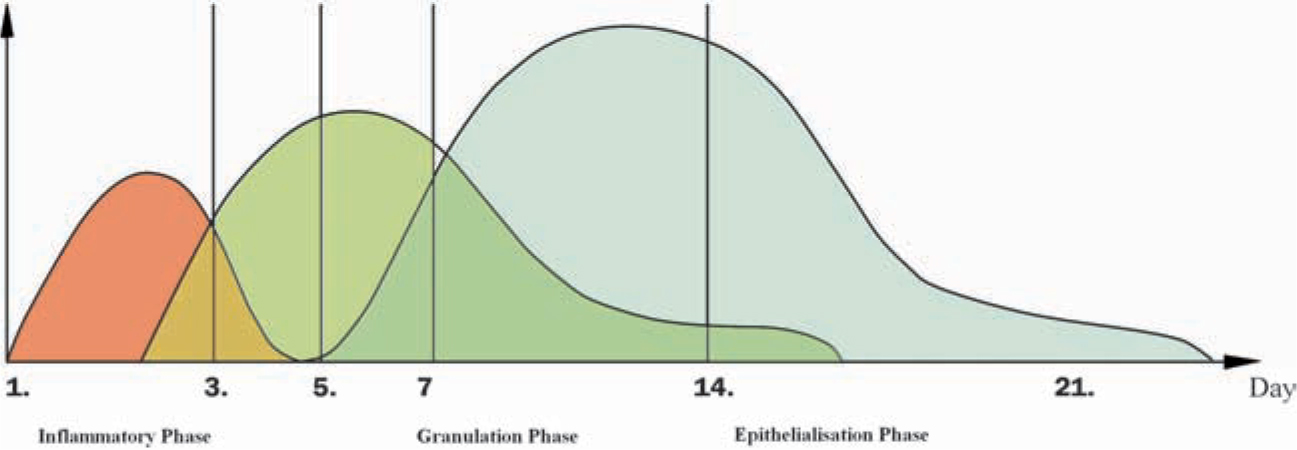
Wound healing occurs in three distinct but overlapping phases, which are referred to as inflamma-tion, proliferation, and regeneration).
Figure 1.1.
Time course and overlapping of the three distinct phases of wound healing. Time is indicated by days after wounding. Figure modified from: Arco G, Horch RE. Chirurgie der Narben. Grundlagen, Prvention und Behandlungsmethoden. CHAZ. 2009; 10:1 German.
Hemostasis and Inflammation (Immediately After Wounding Through Days 46)
Upon wounding, blood vessels are injured, resulting in activation of the endothelium and adjacent platelets followed by vasoconstriction and activation of the coagulation cascade, respectively. A fibrin clot is formed, which consists of fibronectin, thrombin, platelets, and collagen. The importance of the clot is twofold. First, it is a rich source of cytokines and growth factors, which are released as activated platelets degran-ulate.
Chemotaxis and Activation
The influx of neutrophils is enabled through vasodilation, which in turn is caused by prosta-glandins activated through inflammatory mediators released through platelet degranulation and products of proteolysis of fibrin and other matrix components. Interleukin (IL)-1, tumor necrosis factor alpha (TNF-), transforming growth factor (TGF-), platelet factor-4 (PF4), and bacterial degradation products all attract neutrophils into the wound. Infiltration with neutrophils occurs within 24 to 48 h after injury. They do not appear to contribute to the normal healing process other than preventing infection and debriding the wound. Depletion of neutro-phils does not result in significant alteration of the healing process. Subsequently, monocytes are attracted into the wounded area, where they transform into macrophages. The recruitment of macrophages and their activation are essential for effective wound healing; failure to recruit them will result in severely impaired wound healing. They represent the predominant cell type within the wound between 48 and 72 h after wounding. The tasks of macrophages include phagocytosis of expendable neutrophils, pathogenic organisms, cell and matrix debris; mediation of angiogenesis and fibroplasia; and synthesis of nitric oxide (NO). They also initiate the transition to the proliferative phase.
Epithelialization, Angiogenesis, and Provisional Matrix Formation (Day 4 Through 14)
Once activated, macrophages release a multitude of agents at the wound site, thereby initiating the proliferative phase of wound healing: Collag-enases debride the wound; interleukins and TNF stimulate fibroblasts, which initiate granulation, tissue formation, and collagen deposition, TNF- and basic fibroblast growth factor (bFGF) promote angiogenesis; while TGF stimulates kerati-nocytes, which in turn leads to epithelialization. Macrophages also secrete IL-1 and keratinocyte growth factor 2 (KGF-2), which stimulates fibro-blasts to secrete KGF-2 and IL-6, which in turn cause keratinocytes to proliferate and migrate. Keratinocytes are then able to express IL-6 and NO themselves and thereby perpetuate the process. If the basement membrane has been destroyed, epidermal regeneration occurs from proliferating epithelial cells located on the skin edge of the wound. In order to restore the integrity of the epidermal layer, keratinocytes must migrate over the wound margin and therefore cut a path through the fibrin clot or along the interface between the clot and the healthy dermis. For this purpose, leading edge keratinocytes express particularly high levels of tissue-type plasmino-gen activator (tPA) or urokinase-type plasmino-gen activator (uPA), both of which activate plasmin, the chief fibrinolytic enzyme. Various members of the matrix metalloproteinase (MMP) family are also preferentially generated by leading edge keratinocytes as well as fibroblasts, mac-rophages, and monocytes. In particular, MMPs-1, -9, and -10 facilitate migration of the above cells through the extracellular matrix.
The connective tissue in the wound is referred to as granulation tissue because of the granular appearance caused by the invading capillaries. Angiogenesis, that is, the process of forming new blood vessels, is ongoing throughout the previously mentioned phases of wound healing. bFGF and vascular endothelial growth factor (VEGF) are released at the wound site by endothelial cells, macrophages, and keratinocytes. Endothelial cells also generate NO in response to hypoxia, and this in turn stimulates more VEGF production. NO causes vasodilation of the endothelium and has a protective effect on newly formed tissue with regard to ischemia and rep-erfusion injury.