Introduction
Melanoma is the most aggressive form of skin cancer and incidences continue to rise worldwide. According to the American Cancer Society, an estimated 76,100 new cases of melanoma will be diagnosed in the USA and 9710 people are expected to die of metastatic disease [].
Efforts are underway to understand the biology of melanoma heterogeneity to better design strategies for more precise choices for targeting. In this chapter, we review clinical and genetic profiles in melanoma and discuss heterogeneity as one of the most significant causes for cancer therapy resistance.
Clinical and Molecular Classification of Melanoma
Clinical and histological classifications of melanoma have been extensively described (Fig. ] suggesting a fluid transition from one state of cellular differentiation to the other, making it very difficult to trace the origin of melanomas. As tumors become more aggressive, they often lose their pigmentation markers and dedifferentiate acquiring stem cell features, which make them more resistant to therapy.
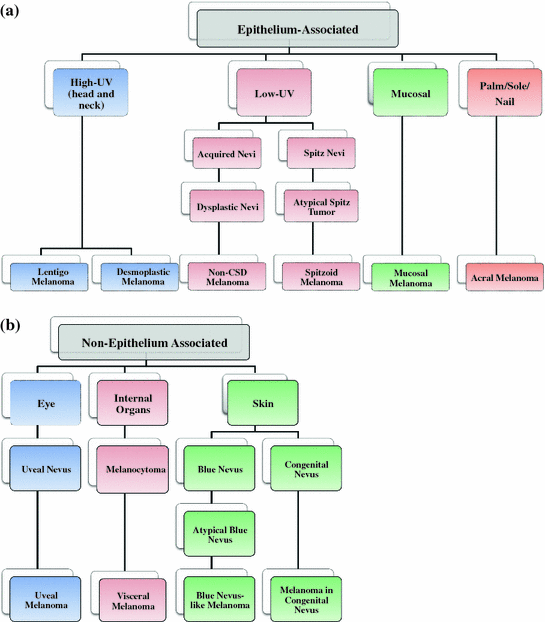
Fig. 1
Clinical grouping of melanoma. There are distinct patterns of clinical appearances of melanoma that led to the distinction of the histogenetic types in the first classification system in 1973. a Melanomas arising from epithelium-associated melanocytes. The relationship between sun exposure and melanoma has been established for decades (high UV melanoma); however, it was later discovered that melanomas can also occur in areas that are well-protected from UV exposure (low UV, mucosal, and palm/sole/nail melanomas). b Melanomas arising from non-epithelium-associated melanocytes. These melanomas fall into the categories of intradermal melanocytic neoplasms (blue nevi, uveal melanoma). These types of melanoma harbor mutations of G protein alpha subunits of Gq family. These mutations are virtually absent from epithelium-associated melanocytes. Melanocytomas are neoplasms of the central nervous system and they closely resemble blue nevi. However, they can pose differential problems to melanoma metastases diagnosis. CSD chronic sun damage. Adapted from Bastian []
The clinical classifications are the results of long-standing observations. They have been critical in making therapy decisions, although their usefulness as guides has been controversial. For practicing oncologists, a distinction between benign and malignant has been most critical, with intermediate stages generating controversial discussions, some of which have been ongoing for decades without a clear resolution. One major reason for this is that any suspected lesion is surgically removed for extensive diagnostic evaluation. Thus, follow-up of existing lesions has rarely been done, leading to considerable variations in risk estimates for dysplastic nevi, as well as biologically early melanomas that progress to aggressive tumors. Genetic analyses of melanocytic lesions have for the first time allowed a more detailed classification, but such new classifications are at an early stage as we know of very few drivers in the disease. Still, the first genetic analyses are becoming routine in making clinical decisions for melanoma therapy.
It is important to discuss the major mutations in melanoma and their affected pathways, while acknowledging that of all human cancers, melanomas carry the most mutations, generally more than 10 per Mb with lung cancer following as the second most non-euploid tumor [). Thus, codrivers can be oncogenes (CDK4, Cyclin D1) or inactivation of tumor suppressor genes (phosphatase and tensin homolog [PTEN]). Likely, cells carry more than one codriver, but their real number remains uncertain and will only be better understood in years to come. Gaining this knowledge is important because each codriver will likely constitutively activate additional pathways, which will require specific targeting for therapy.











