Springer-Verlag Berlin Heidelberg 2014
Raymond L. Barnhill , Michael W. Piepkorn and Klaus J. Busam (eds.) Pathology of Melanocytic Nevi and Melanoma 10.1007/978-3-642-38385-4_1
1. Melanocytes
Among the somatic cells, the differentiated melanocyte has the hallmark property of pigment production in the skin, hair, and eye. That pigment is a biopolymer termed melanin, which serves unique physiological functions in skin biology. The importance of the functions relates to photoprotection, inflammation, aging, and mitigation of neoplastic stimuli. Herein, we discuss general elements related to melanocytic development, biological function, and melanocytic neoplasia.
1.1 Embryology and Histogenesis of Melanocytes
The melanocyte is the specialized and differentiated progeny of the melanoblast, the conceptual melanocyte stem cell (melSC) that is considered to be of neural crest origin based, in part, on in vivo studies of ablation and transplants of the neural crest that produce changes in patterns of cutaneous pigmentation []. It gives rise to a wide variety of cellular derivatives migrating peripherally to generate anatomically and functionally diverse structures. Migration of the melanoblasts, one of these lineage-restricted stem cells, from the neural crest to sites of colonization in the epidermis and the follicular epithelium, as well as focally to other sites such as the heart and central nervous system, gives rise to the pigmentary system.
Experimentally, cell type-specific markers are used to identify the putative stem cell; although it has been challenging to verify sensitive and specific markers for this purpose, Tryp-2 (tyrosinase-related protein 2)/Dct (dopachrome tautomerase) is used as an operational marker for early melanoblasts [].
The temporal stage for commitment, also known as specification, of stem cells to the pathway of melanocytic differentiation is not resolved and may be species dependent [].
During development of human skin and following the expression of a number of genes including the HOX and Wnt loci, differentiated melanocytes appear first in the vicinity of nerves and blood vessels of the dermis at approximately 410 weeks of fetal life [].
While initially melanocytes are randomly distributed throughout all epidermal layers [].
1.2 Anatomic Distribution of Melanocytes
The majority of somatic melanocytes reside in the skin, in particular within the epidermis and hair follicles; some of them reside in the dermis and may be found around sebaceous glands or near the lactiferous ducts of the nipple [].
Epidermal melanocytes are present in all regions of the integument. Estimations of the volume of melanocyte mass in an adult human range between 1 and 1.5 ml [].
1.3 Morphology of Melanocytes
1.3.1 Light Microscopy
Melanocytes of the human epidermis are territorially distributed at a regular distance from each other along the dermoepidermal junction, resulting in an approximate ratio of 1 melanocyte per 410 basal keratinocytes in two-dimensional histologic imagery [). Their dendrites are usually invisible in HE-stained paraffin sections, unless they are packed with melanin. The dendrites maintain intercellular contacts with epidermal keratinocytes and with dermal fibroblasts. In hair follicles, melanocytes are located at the epithelial side of the basement membrane bordering the central hair papilla.
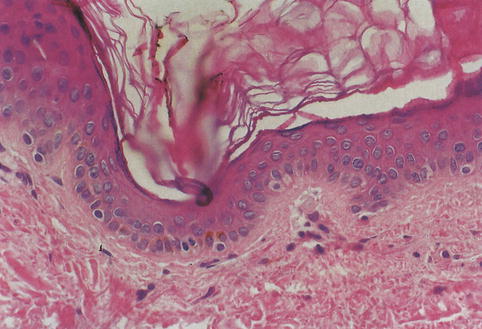
Fig. 1.1
Epidermal melanocytes. Solitary cells with pale cytoplasm and round or ovoid nuclei localize at the dermoepidermal junction, just below basal layer keratinocytes
The nucleus of the melanocyte, whose contour is polygonal or indented, is somewhat smaller and more hyperchromatic than the nuclei of nearby keratinocytes [).
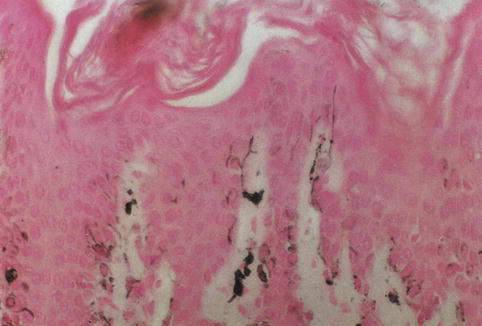
Fig. 1.2
Silver-stained melanocytes. The dendritic processes of melanocytes become apparent (Masson Fontana)
Resident epidermal melanocytes in adults may proliferate in response to ultraviolet light or growth factors released during tissue regeneration []. Lentiginous melanocytic hyperplasia refers to a single-cell unit pattern of melanocytic proliferation along the dermoepidermal junction. Nesting denotes a clustering of proliferative melanocytes. Single-cell, dyscohesive growth present throughout the entire epidermis defines the pagetoid pattern of proliferation. Lentiginous hyperplasia is often a feature of lentigines or lentiginous forms of melanocytic nevi. Nesting is typical of melanocytic nevi. Pagetoid spread, although often associated with melanoma, may also be observed follow-ing epidermal injury and in both acquired and congenital nevi of children, Spitz nevi, pigmented spindle cell nevi, and common acquired nevi of acral skin. The pattern of benign melanocytic proliferation is typically symmetrical and shows in vertical growth a gradient of cytological development with dermal descent, which is referred to as maturation.
1.3.2 Electron Microscopy
By electron microscopy, melanocytes typically have a prominent Golgi complex and rough endoplasmic reticulum as well as numerous mitochondriafeatures characteristic of metabolically active cells. Melanocytes lack tonofilaments and desmosomes but contain intermediate filaments (vimentin). Where melanocytes appose the epidermal basal lamina, half-desmosome-like structures may form [].
The ultrastructural hallmark of melanocytes is a unique organelle, the melanosome [].
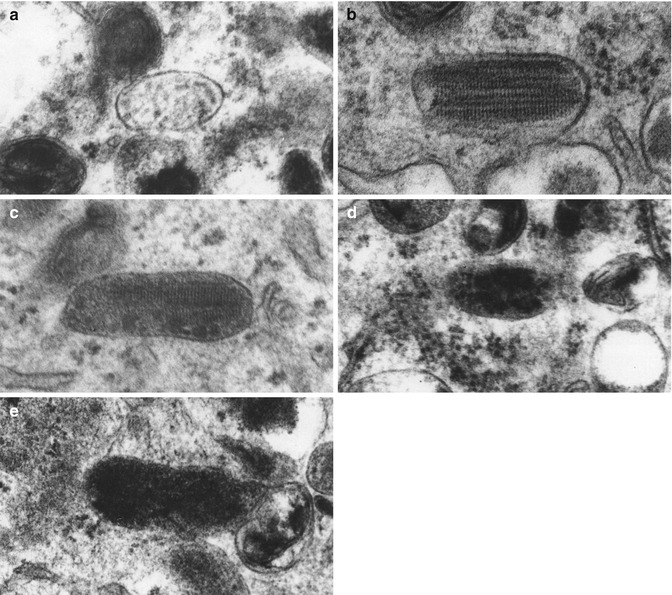
There are four stages in the formation of eumelanosomes [].
Fig. 1.3
Ultrastructure of eumelanosomes. The four stages of eumelanosome formation are illustrated in electron micrographs. Stage I: vesicular inclusions ( a ; 100,000); stage II: striated inclusions ( b ; 100,800); early stage III: granular deposits on striated inclusions ( c ; 130,100); late stage III: more granular deposits ( d ; 94,000); stage IV: fully melanized organelles ( e ; 96,000) (Courtesy of Dr. M. Seiler, West Roxbury VA Medical Center, West Roxbury, MA)
The development of phaeomelanosomes also resolves itself into four stages []. Phaeomelanosomes are round rather than oval throughout all stages. In stage I, a vacuole is present, containing small microvesicles. These microvesicles become more numerous in stage II. Deposition of melanin pigment on microvesicles and in the matrix characterizes stage III. Stage IV occurs when the matrix is completely masked with melanin.