


To my wife, Frederika, and our own special chemisty.

In 1675, Sir Isaac Newtonalready celebrated as a scientific genius in his own lifetimemodestly wrote, If I have seen further it is by standing on the shoulders of Giants. He was referring to the pioneering work of scientists in the preceding centuries, and how he built on their knowledge and discoveries to produce his own scientific contributions.
Well, as you read the following pages youll join me in standing on Newtons shoulders and quite a few more, besidesthe broad shoulders of Galileo, Grace Flyer, Paracelsus, Robert Boyle, Madame Curie, Joseph Priestly, and Dmitri Mendeleev, to name just a few. Some of them devoted their entire lives to science; others gave up their lives for the same cause.
Luckily, those who helped guide this particular book into your hands are still with us, and they deserve my gratitude for their guidance and patience. They include my agent, Jim Levine of the Levine Greenberg Rostan Literary Agency, Workman editor and brother in arms, Danny Cooper, and the Workman team responsible for helping my words take shape on the pageproduction editor Beth Levy, production manager Steven Bucsok, typesetter Barbara Peragine, designer Gordon Whiteside, and illustrator Cara Bean.
The following individuals and organizations have also played a part in producing this book, either through direct consultation or providing yet more shoulders for me to stand on: The Bath Royal Literary and Scientific Institution, Berkshire Film & Video, Frank Ciccotti, Gregory Etter, Dr. Gary Hoffman, Dr. Peter Lydon, MITs Educational Studies Program, Peter Rielly, Elizabeth Stell, Williams College Astronomy Department, and Woods Hole Oceanographic Institution.
Contents
: Get to Know an Atom
: Hey, Cool It!
: A Star Is Boron
: Good Conduct Award
: Fixin to Use Some Nitrogen
: Global Cooling
: On the Trail of Cavendish
: Try the Fluorine Tooth Test
: Even Neon Gets Excited
: Hot Ice
: Trick Birthday Candles
: Crackpot Crystal
: CursesFoiled Again!
: Dont Sand So Close to Me
: Fast-Track Fossils
: Playing Cat Detective
: Save That Silver!
: Ill Have an Egg Float
: Not Such a Softie
: Burning Iron
: May the Force Be with You
: Did You Wind the Potato Clock?
: Is That a Tin Tin Can?
: Because Youre Worth It
introduction
Matter Really Matters
Think about that word matter for a moment. Do you have any idea what it means? Is everything some sort of matter? Or maybe some stuff is and other stuff is something else? Or could it be that things are made up partly of matter, with other things, like forces, mixed in? Maybe none of these answers is even close? Or the answer is just too darned complicated to find? Does it even really matter?
The answer is 100 percent YES. It does matter, and best of all, finding out how and why is a whole lot of fun. Thats what this book will do. Its going to show you how everything is matter, but more than that, it will help you see the world around you in a whole new way. Youll understand why things look the way they do (or why they sometimes cant be seen), why they behave the way they do, and what can and cannot be changed about them.
The following pages will take you on a chemistry journey through the inner workings of all matter. When we think of matter, we think of thingsdoughnuts, car tires, cell phones, and pineapples. They all seem different, but if we look more closely, we learn that those familiar examples share certain elements; its just that those elements are put together in different ways.
And its that word, elements, that is the key to understanding matter. Once you start separating the bits that make an apple an apple or a pencil a pencil, you eventually get to a point where you cant do any more separating. You find the basic building blocks of matter, the things that can be arranged in all sorts of awesome ways to make the world around us. These are the elements, the end of the line, beyond which its impossible to reduce things into simpler structures.
That is the world this book inhabitsthe world of elements. Youll see that these elements are all atoms, but atoms differ from one another in particular ways. The differences come from the tiny particles that all atoms have, but in different numbers and arrangements. The number of protons (the positively charged particles) is the main ID check of an element. The protons are all in the nucleus, or center, of each atom. And just to make things interesting, you might also find neutrons inside some of these atoms, too. Neutrons have no charge, but they do have mass (weight)... and that can make elements behave differently.
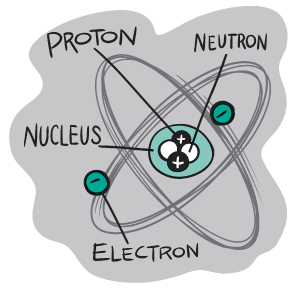
Things really get going when you come across the negatively charged electrons, which orbit the nucleus a little bit like planets orbiting the Sun. Just as you might have noticed with electricity or magnets, opposites attract, so the negative charge of one element might attract the positive charge of another... leading to some pretty cool connections. Electrons are the fidgety members of the element dramathey might stay put and attract other elements, or jump off to a different element, or even share time between two elements. At this point, you come to learn one of the keys to all science: The behavior of electrons dictates so much of what we call chemistry.
Setting the Table
Just how do those elements get along with each other? Its easy to see that gold looks different from carbon and each of those is unlike aluminum or oxygen. Whats harder to see is that the elements arent all out in the world behaving uncontrollablyunlike your screwball brother or sister, theres a way to predict their behavior. How? Thats where the periodic table of elements comes into play.
The periodic table is a road map of all the matter in the universe. It arranges the elements according to the particles that make them up. Since the main ID for an element is its number of protons, the table begins with hydrogen (which has one proton, so its atomic number is 1) and ends with oganesson, which has an atomic number of 118. You probably hadnt ever heard of oganesson before a few seconds ago, but now you can probably work out how many protons it has!

Weve gone pretty easily from 1 to 118, but why are the elements arranged in rows and columns? That takes us back to those orbiting electrons. Think again of electron orbits, which scientists call shells or energy levels. Well, atoms have seven possible electron shells. Once the innermost shell (which can hold two electrons) has been filled up, electrons move to the second shell, and when thats filled, they start in on the third, and so forth. And each shell can hold more electrons than the previous one. But take another look at the periodic table. Notice how that bigger top chunk has seven rowsthats because there are seven possible electron shells. So the elements in the first row have one electron shell, those in the second have two, and so on, right up to the seventh row.
Next page





















