Selenium is incorporated into protein through the use of highly specialized, evolutionary conserved machinery. The chapters in this section focus on the molecular components involved in biosynthesis of the 21st proteinogenic amino acid, selenocysteine, and its incorporation into protein.
Springer Science+Business Media, LLC 2016
Dolph L. Hatfield , Ulrich Schweizer , Petra A. Tsuji and Vadim N. Gladyshev (eds.) Selenium 10.1007/978-3-319-41283-2_1
1. Selenocysteine tRNA[Ser]Sec: From Nonsense Suppressor tRNA to the Quintessential Constituent in Selenoprotein Biosynthesis
Abstract
When selenocysteine (Sec) tRNA[Ser]Sec was originally discovered, it was proposed to be the first nonsense suppressor tRNA found in mammalian and avian tissues, since it exclusively decoded the nonsense codon, UGA, which normally dictates the cessation of protein synthesis. This tRNA was subsequently shown to be Sec tRNA, which inserted Sec into protein as the 21st proteinogenic amino acid. Once it was established that this tRNA was aminoacylated with serine by seryl-tRNA synthetase and served as the scaffold for Sec synthesis, Sec tRNA was appropriately named Sec tRNA[Ser]Sec. The mammalian Sec-tRNA[Ser]Sec population consists of two isoforms that differ from each other by a single 2- O -methyl moiety on the uridine at position 34, designated Um34. The non-Um34 isoform is involved in the synthesis of a subclass of selenoproteins, called housekeeping selenoproteins, while the Um34 isoform supports synthesis of stress-related selenoproteins. These novel functions and other unique features of Sec tRNA are the subjects of this chapter, supporting the idea that this tRNA is the quintessential constituent in selenoprotein biosynthesis.
1.1 Introduction
Selenocysteine (Sec) tRNA was discovered in 1970 when a seryl-tRNA was found to form phosphoseryl-tRNA [).
1.2 Primary and Secondary Structures of Sec tRNA
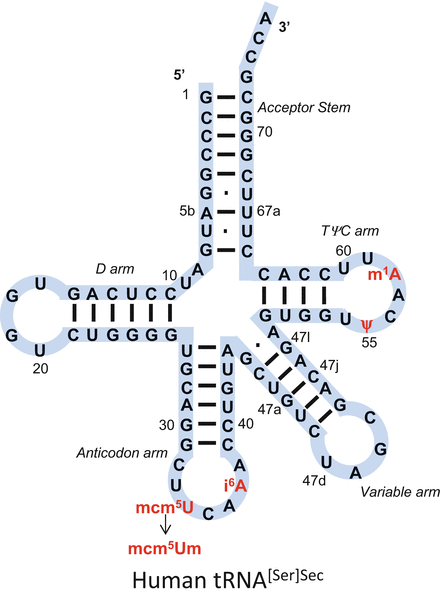
Since its discovery as a seryl-tRNA [], which differed by a single 2- O -methylribosyl at position 34, designated Um34. The highly modified base at position 34 is 5-methoxycarbonylmethyluridine (mcm5U), while the nucleoside at this position is 5-methoxycarbonylmethyl-2- O -methyluridine (mcm5Um) . Interestingly, the mammalian isoforms are 90 nucleotides long making them the longest tRNAs sequenced in higher vertebrates. Another unique feature about these tRNAs is that they have relatively few modified bases, and thus, are highly undermodified compared to all other known tRNAs that normally contain 1517 amended bases.
The secondary structures of the two isoforms are shown in Fig. ]. The D-stem of Sec tRNA has more base pairs, five to six, than all other tRNAs, which have three to four. Furthermore, Sec tRNA does not have the dihydrouracil base found in the D-loop in other tRNAs. The long variable arm and the extra base in the acceptor/TC stems account for the bases that make this tRNA much longer than canonical tRNAs. When comparing Sec tRNA to all other tRNAs, it is indeed the most unique adaptor RNA described to date. These features account for the inability of elongation factors TU or 1alpha to bind tRNA[Ser]Sec and the requirement instead for dedicated elongation factors SelB and EF-SEC in bacteria and eukaryotes, respectively.
Fig. 1.1
The primary structure of human tRNA[Ser]Sec is shown in a cloverleaf model. There are 90 bases in mammalian tRNA[Ser]Sec and the bases are numbered as shown in the figure (see also []). The acceptor stem constitutes the paired 5 and 3 terminal bases, the D stem and loop constitute the six paired and four unpaired bases of the left portion of the tRNA, the anticodon stem and loop, the six paired and seven unpaired bases of the lower portion of the tRNA, the variable stem and loop, the five paired and four unpaired bases, and the TC stem and loop, the four paired and seven unpaired bases of the right portion of the tRNA. Mammalian tRNA[Ser]Sec contains base modifications at positions 34 (mcm5U), 37 (i6A), 55 () and 58 (m1A) as described in the text. The two isoforms differ from each other by a single methyl group on the 2- O -ribosyl moiety at position 34
Sec tRNA is rightfully named Sec tRNA[Ser]Sec, since it is initially aminoacylated with serine (Ser) by seryl-tRNA synthetase (SERS); but as a result of synthesizing Sec from Ser on the tRNA, it inserts Sec into protein. Historically, tRNAs were named by the amino acid attached to them by their corresponding aminoacyl-tRNA synthetase, and unlike any other known tRNA in eukaryotes, Sec is synthesized directly on its tRNA. The novelty of these events are highlighted by the uniqueness of its name, tRNA[Ser]Sec.
1.3 Um34 Addition to Sec tRNA[Ser]Sec, a Most Highly Specialized Modification
In the maturation of Sec tRNA[Ser]Sec, the final modification is the addition of Um34, which is indeed a highly specialized event. For example, its synthesis depends on the secondary and tertiary structure of Sec tRNA [].
An unresolved question regarding Um34 addition to the mcm5U isoform is when exactly does this addition occur during Sec tRNA[Ser]Sec maturation. Synthesis of the modified bases, and m1A at positions 55 and 58, respectively, occurs in the nucleus, while the synthesis of mcm5U occurs in the cytoplasm []. Thus, it appears that i6A must be present for Um34 synthesis in vivo. These observations raise questions as to where in the cell and at what stage during tRNA[Ser]Sec maturation do Um34 synthesis take place in vivo, if the TRIT1 reaction is poorly reversible and Um34 cannot be synthesized on mcm5U lacking i6A?
Sec-tRNA[Ser]Secmcm5Um is essential for stress-related selenoprotein synthesis and the presence of Um34 [].
1.4 Trsp , the Sec tRNA[Ser]Sec Gene
The Sec tRNA[Ser]Sec gene is designated Trsp . It is a single copy gene in all organisms examined except zebrafish, which has two gene copies [].
1.5 Transcription of Trsp
Trsp is transcribed by Pol III as are all other eukaryotic tRNAs (reviewed in []. The 5-triphosphate at the first nucleotide in Sec tRNA[Ser]Sec remains intact through maturation of the tRNA, but its possible role in Sec biosynthesis and incorporation into protein has not been resolved.