Nonproliferative Diabetic Retinopathy
Diabetes is an epidemic affecting more than 18 million people in the United States (). The retinal manifestations of diabetes mellitus are broadly classified as either nonproliferative diabetic retinopathy (NPDR) or proliferative diabetic retinopathy (PDR).
Nonproliferative diabetic retinopathy occurs when there are only intraretinal micro-vascular changes, such as altered retinal vascular permeability and eventual retinal vessel closure. Clinically, the hallmark of the nonproliferative phase is microaneurysms and intraretinal abnormalities. Neovascularization is not a component of the nonproliferative phase. However, in advanced NPDR, nonperfusion of the retina may develop and lead to the proliferative phase. Proliferative diabetic retinopathy is characterized by new vessels and sometimes fibrous bands proliferating on the retinal surface. In both nonproliferative and proliferative diabetic retinopathy, macular edema can occur as increased retinal vascular permeability leads to accumulation of fluid in the retinal area serving central vision. This chapter focuses on the clinical aspects of NPDR.
Pathophysiology Of Nonproliferative Diabetic Retinopathy
Effective and appropriate management of NPDR is dependent on a clear understanding of the disease course. Chronic hyperglycemia in poorly controlled diabetes results in biochemical alterations and altered hemodynamics of the retinal vasculature, which lead to chronic hypoxia (). Since the retina is a highly metabolic tissue dependent on optimal oxygenation, compensatory pathways, such as upregulation of vascular endothelial growth factor (VEGF) protein, are targeted against this retinal hypoxia. These efforts are futile, however, and ultimately result in the pathologic processes of NPDR: retinal capillary microaneurysms, vascular permeability, and eventual vascular occlusion, or capillary closure.
Inflammatory Mechanisms
Increasing evidence suggests that inflammation may play a role in the pathogenesis of diabetic retinopathy. Multiple animal and human tissue studies have indicated that chronic inflammation contributes to diabetic vascular damage.
Intercellular adhesion molecule 1 (ICAM-1), a member of the immunoglobulin superfamily involved in immune activation and inflammation, and its counter-receptor CD18 are thought to play a pivotal role ().
Studies of human tissue have also suggested an inflammatory role in the pathogenesis of diabetic retinopathy. Immunoassays for the quantitative determination of soluble ICAM-1 in the vitreous of PDR patients undergoing vitrectomy showed elevated ICAM-1 levels when compared with that in the control group (). Similar to the animal studies, these human studies suggest that inflammation may play a central role in the development of diabetic retinopathy.
While the precise components of the inflammatory pathways in the pathogenesis of diabetic retinopathy are still being investigated, the recognition of the role of inflammation in this retinal disease suggests the potential utility of using anti-inflammatory therapies. Further research is required to translate these scientific findings into clinical care.
Microaneurysms
The retinal capillary microaneurysm usually is the first visible sign of diabetic retinopathy. Microaneurysms, identified clinically by ophthalmoscopy as deep-red dots varying from 15 to 60 m in diameter, are most common in the posterior pole. Although microaneurysms can be associated with other retinal vascular diseases, particularly those associated with vascular occlusion such as branch and central vein occlusions, they are the hallmark of NPDR.
Histologically, microaneurysms are hypercellular saccular outpouchings of the capillary wall, as demonstrated by trypsin digest retinal mounts ().
The mechanism for the formation of microaneurysms is also unknown. Possible mechanisms include release of a vasoproliferative factor with endothelial cell proliferation, weakness of the capillary wall (from loss of pericytes), abnormalities of the adjacent retina, and increased intraluminal pressure ().
Microaneurysms may be difficult to differentiate from punctate hemorrhages seen in diabetic retinopathy. However, on the early frames of a fluorescein angiogram, micro-aneurysms are easily distinguished from intraretinal hemorrhages because they exhibit bright hyperfluorescence against the darker choroidal background, whereas retinal hemorrhages block fluorescence (Figs. ). When the number of microaneurysms increases, there is an increased likelihood that the other microvascular changes of diabetic retinopathy may also be present.
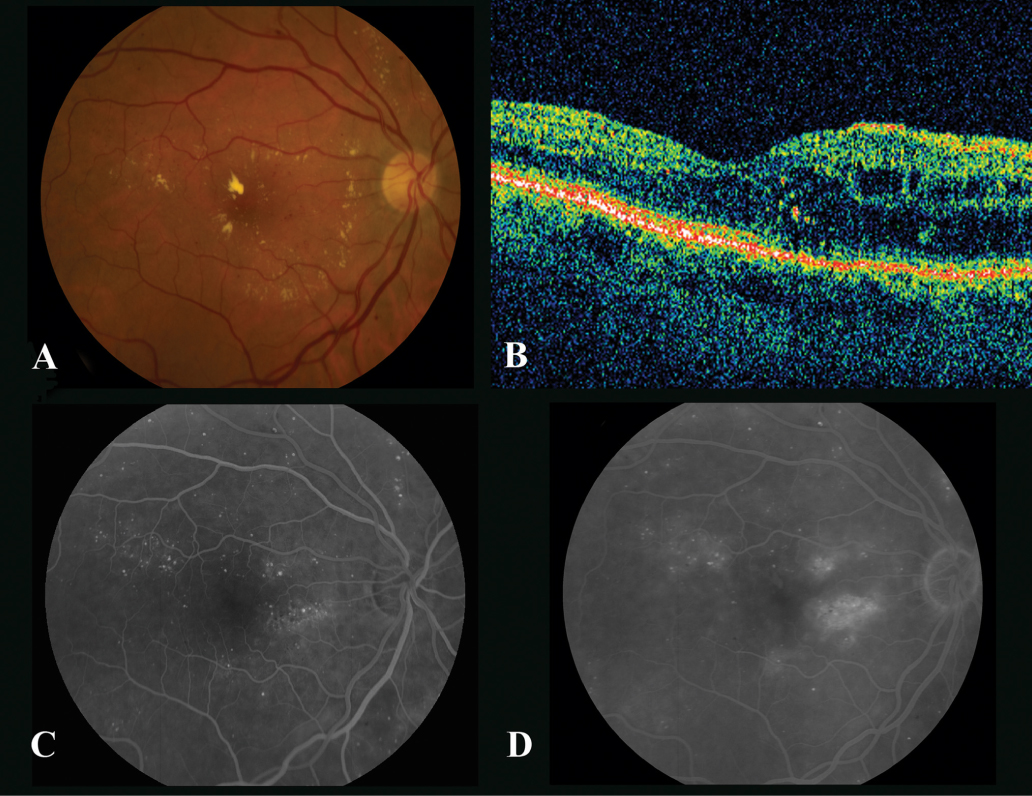
Fig. 1.
The right eye of a 55-year-old woman with mild macular edema. ( A ) Color photograph shows circinate ring of lipid and microaneurysms. Best-corrected vision is 20/25. ( B ) Optical coherence tomography shows mild macular edema with preservation of the foveal contour. ( C ) Early phase of fluorescein angiogram highlights the multiple microaneurysms. ( D ) Late phase of fluorescein angiogram shows patchy areas of leakage.
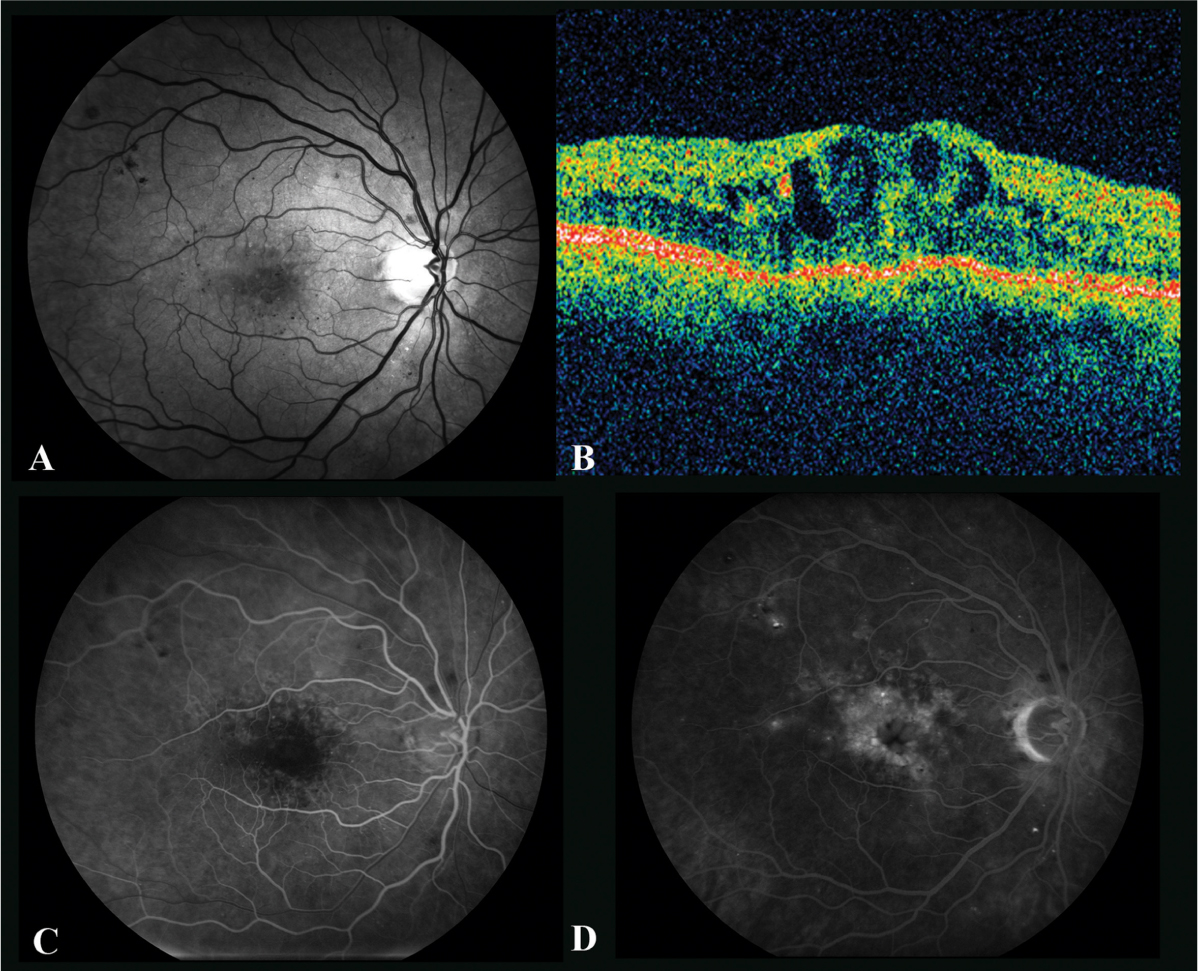
Fig. 2.
The right eye of a 68-year-old man with chronic macular edema. ( A ) Red-free photograph shows multiple microaneurysms in this patient with type 2 diabetes for 45 years. Best-corrected visual acuity was 20/63 despite multiple treatments over many years, including focal laser, subtenon triam-cinolone, and intravitreal bevacizumab. ( B ) Optical coherence tomography shows large intraretinal cysts and foveal distortion. ( C ) Early fluorescein angiogram highlights the multiple microaneurysms. ( D ) Late fluorescein angiogram shows petalloid edema.
Vascular Permeability
As microvascular damage increases in the presence of excessive blood glucose, increased vascular permeability occurs through multiple pathways. Vascular endothelial growth factor (VEGF) protein is thought to play a pivotal role. A healthy human retina contains little VEGF, but its level is increased in response to hypoxia that can occur in states such as diabetic retinopathy. Originally described as vascular permeability factor, VEGF is not only a mediator of new blood vessel formation seen in PDR, but also an inducer of vascular permeability, which can lead to retinal edema seen in both nonpro-liferative and proliferative diabetic retinopathy ().