Part I
Materials and Nanomaterials
Springer Nature Singapore Pte Ltd. 2017
Raj Mohan B. , G. Srinikethan and Bhim Charan Meikap (eds.) Materials, Energy and Environment Engineering 10.1007/978-981-10-2675-1_1
Characterization of Citrus Peels for Bioethanol Production
John Indulekha 1
(1)
Department of Chemical Engineering, National Institute of Technology Tiruchirappalli, Tiruchirappalli, Tamil Nadu, India
Appusamy Arunagiri (Corresponding author)
Email:
Keywords
Lignocellulose Biofuel Citrus peels NDF ADF Lignin
Introduction
The increasing greenhouse gas emissions, acid rain, climate change and fossil fuel depletion have given rise to interest in clean energy. Biomass has greater potential for renewable and sustainable source of energy. Biomass is the fourth largest energy source around the world. One way to reduce the crude oil consumption as well as environmental pollution is to produce biofuels from biomass (Demirabs ). Any lignocellulosic material is composed of cellulose, hemicellulose and lignin. It is always suggested to opt for a biomass which contains low lignin and high carbohydrates for better conversion. In this aspect, citrus peel waste (CPW) is an attractive feedstock of lignocellulosic biomass which can turn into bioethanol. When compared with other lignocellulosic materials, pectin is a significant component in citrus peels. Pectin is a value added product which will increase the interest in the utilization of citrus peel.
Especially in India, citrus peels are not much explored for bioethanol production as it is for other lignocellulosic biomass like sugarcane bagasse or rice straw. This work aims at the significance of characterization of citrus peels such as orange peel and sweet lime peel for biofuel production. The several characteristics of citrus peels were investigated through the proximate analysis, ultimate analysis, higher heating value, fourier transform infrared (FTIR) analysis. The rate of volatiles evolution and mass loss due to thermal degradation were determined by the thermogravimetric (TG) and derivative thermogravimetric (DTG) analysis. On the basis of these analyses the potential of citrus peels as energy source has been discussed.
Materials and Methods
2.1 Materials
Citrus peels were collected from local juice shop in the campus, NIT Trichy, India. The collected citrus peels were washed properly with distilled water and then sun-dried. Sun-dried samples were stored air tight in polyethylene bag. Biomass was further powdered using grinder and then sieved using IS sieves. Peels of particle size 1 mm was selected for the characterization studies (Fig. ).
Fig. 1
Photograph of sweet lime peel a before grinding b after grinding
2.2 Experimental
Proximate analysis was carried out to estimate moisture content, ash, volatile matter and fixed carbon by difference. This analysis was done according to Bureau of Indian Standards code IS:1350-1 (1984). Ultimate analysis was used to determine the elemental composition such as carbon, hydrogen, nitrogen, sulfur and oxygen by difference. Bomb calorimeter was used to determine the higher heating value (HHV). The lignocellulosic composition that is hemicellulose, cellulose, lignin and extractives were determined by the analytical method given by Van Soest (). The functional group present in the lignocellulose was determined using FTIR spectrometer (Perkin Elmer). Within the range of 4000400 cm1 in absorbance mode was selected for FTIR analysis of the citrus peels. Thermal analysis, such as TG (Thermo-gravimetric) and DTG (Derivative thermo-gravimetric) were performed by a thermal analyzer (Perkin Elmer). Almost 10 mg of the material was used for analysis in the atmosphere of nitrogen as sweeping gas with purge rate of 20 ml/min from 30 to 950C at heating rate of 10C/min.
Results and Discussion
3.1 Proximate and Ultimate Analysis
The proximate and ultimate analysis of orange and sweet lime peels are shown in Table . Proximate analysis results exhibit the weight percentage of moisture, ash, volatile matter and fixed carbon by difference. The presence of high moisture in biomass requires prior drying to remove the moisture and reduces the energy efficiency of the system. The volatile matter content of these peels are high which revealed that it can also be utilized for the pyrolysis process because biomass with more volatile matter is highly reactive and also get devolatilized easily than that of the lesser volatile matter and producing less fixed carbon. The ash in the biomass is low in comparison to other biomass. Ultimate analysis of orange peel and sweet lime peel shows carbon, hydrogen, nitrogen, sulphur and oxygen by difference. This result proves that the nitrogen and sulfur content in the peel is very little, hence this peel produces the lowest measure of nitrogen and sulfur oxides in thermo chemical transformation process. Good amount of carbon, hydrogen and oxygen can lead to higher ethanol yields. Higher heating value of sweet lime peel is 14,853 kJ/kg and for orange peel is 15,690 kJ/kg.
Table 1
Proximate and ultimate analysis of citrus peels
Proximate analysis (wt%) |
|---|
Property | Orange peel | Sweet lime peel |
|---|
Moisture | 4.95 | 4.89 |
Volatile matter | 81.52 | 76.89 |
Fixed Carbon* | 7.02 | 10.40 |
Ash content | 6.50 | 7.82 |
Ultimate analysis (wt%) |
Carbon | 43.13 | 31.50 |
Hydrogen | 5.30 | 3.95 |
Oxygen* | 50.11 | 63.74 |
Nitrogen | 1.41 | 0.78 |
Sulfur | 0.05 | 0.03 |
*by difference
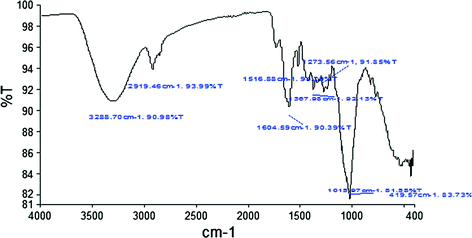
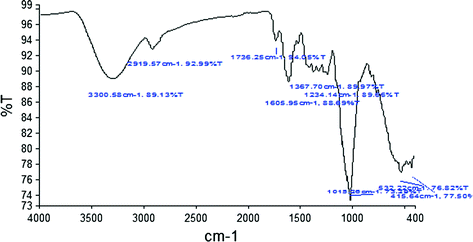
3.2 FTIR Spectroscopy
The FTIR technique is utilized to analyze the chemical components and distinctive functional group. Figures ).
Fig. 2
FTIR spectra of orange peel
Fig. 3
FTIR spectra of sweet lime peel
Table 2
Functional group present in citrus peels from FTIR spectroscopy
Wavenumber (cm1) | Bond | Composition |
|---|
3333 | OH stretching | Alcohol and pectic acid |
2921 | CH Asymmetrical stretching | Lignin |
1633 |