1. Chemistry of Bulk Sweeteners
Sweeteners are the primary ingredients in the manufacture of confections . Chemically, the primary sweeteners in confections are carbohydrates, which consist of a group of widely varied chemical substances present in both plants and animals. For example, in dry corn, approximately 55% of the solids are carbohydrates. The word carbohydrates itself means hydrated carbon. Thus, carbohydrate chemistry mostly deals with chains of carbon atoms hydrated with water, with a general formula of Cx(H2O)y.
Although only three atoms are involved, the chemistry of sweeteners can be very complex. Variations such as carbon chain length and branching, among others, allow for the existence of numerous combinations of the three atoms that provide the range of chemical characteristics of carbohydrates. The main groups of interest for confectionery manufacture are monosaccharides (e.g., glucose and fructose), oligosaccharides (e.g., the disaccharides including sucrose, maltose, and lactose, and starch hydrolysates), and polysaccharides (e.g., starches). Oligosaccharides are carbohydrates consisting of 220 monosaccharide units joined by glycosidic linkages. Compounds containing three saccharides are trisaccharides, while four to ten are tetra-, penta-, hexa-, hepta-, octa-, nona-, decasaccharides, respectively. Various oligo- and polysaccharide products are used in confections. However, sweetening power generally decreases as carbon chain length increases (although other factors affect sweetness as well). Most confections contain primarily smaller saccharides, but with a balance of oligosaccharides for functional properties (i.e., control of crystallization ).
A wide range of sweeteners is used in the manufacturing of confections and chocolates, with the most common being cane or beet sugar (sucrose), glucose (corn) syrup, high fructose syrup, liquid sugar (67% sucrose dissolved in water), and invert sugar, the hydrolysis product of sucrose. Sugar alcohols (or polyols) are used for diabetic candies since they induce no insulin response. High intensity sweeteners are used to enhance sweetness, particularly when sugar alcohols are used.
The original candie s probably were sweetened naturally, with materials like honey and maple syrup. As sucrose became more available, confectioners began using it as the primary sweetener in confections, with its hydrolysis product, invert sugar, used as the first doctoring agent (to control crystallization). As the sweetener industry matured, more and more different types of sweeteners have become available that provide excellent control for the confectioner. Knowledge of the physical and chemical properties of carbohydrates has made it possible for the confectionery industry to develop the many products available on the market today.
Sweeteners, beyond providing sweetness to confections, play an important role in determining the texture . Crystallization of sucrose in some products is encouraged, while in others, complex carbohydrates such as glucose (corn) syrup are added to control or prevent crystallization. Chemical differences between carbohydrates similarly can result in different product characteristics. For example, the color of a number of candies is impacted by the choice of sweetener used in manufacturing. Sweeteners also aid in moisture control. Properly chosen, they either prevent moisture loss or moisture uptake. In addition, they add bulk in candies. The following sections discuss the basic chemistry of saccharides and some of their chemical properties. Their physical properties are the subject of Chapter .
1.1 Monosaccharides
Saccharides are composed of three basic building blocks , a carbon chain (CCC) , hydroxyl groups (OH) , and either an aldehyde (CHO) or ketone (CH COCH ) group. The chain length for monosaccharides can vary from three carbons in the simplest sugar to as many as seven. The monosaccharides most important to the confectionery industry are those containing six carbons (glucose, fructose). The presence of either an aldehyde or a ketone group in glucose and fructose gives rise to two categories known as aldose and ketose sugars. The structural difference between the two six-carbon sugars is that glucose contains an aldehyde group while fructose contains a ketone group.
The three most important monosaccharides in candies are glucose, fructose and galatose because their free aldehyde group makes them reducing sugars (defined later) and therefore, they can participate in the browning reactions. In older literature, glucose and fructose are referred to as dextrose and levulose, respectively. These terms arise from the direction in which a plane of polarized light is rotated when passing through a solution of each sugar: glucose rotates a plane of light to the right (dextro) and fructose rotates a plane of light to the left (levulo).
The word dextrose is still used in describing dextrose equivalent (DE), an important property that describes the reducing capacity of starch hydrolysate syrups . The confectionery industry uses the term dextrose when pure glucose is used. The term glucose is often used to describe the hydrolysate product of starch, more correctly called starch hydrolysate or glucose syrup.
1.1.1 Glucose/Dextrose
Glucose is widely distributed in nature, including the blood of animals and in the sap of plants. It also forms the base unit in one of the most important polysaccharides, starch, and is an important component of confectionery sweeteners .
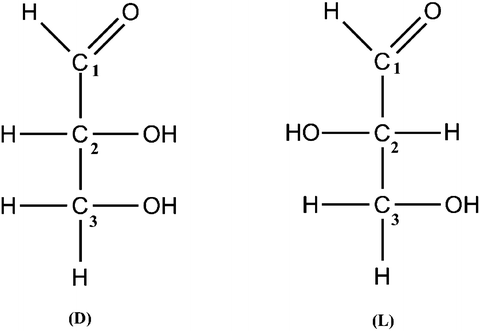
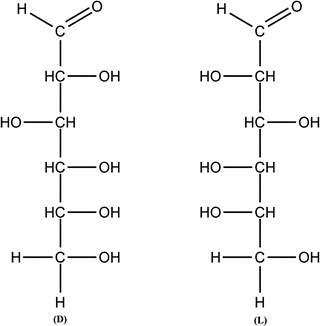
Glucose contains asymmetric carbons, those that have four different substitute groups attached, which gives rise to two isomers, designated as D or L glucose. The structure of D-glucose is shown in Figure . Among this family of monosaccharides, glucose, galactose and xylose are of particular interest to the confectionery industry.
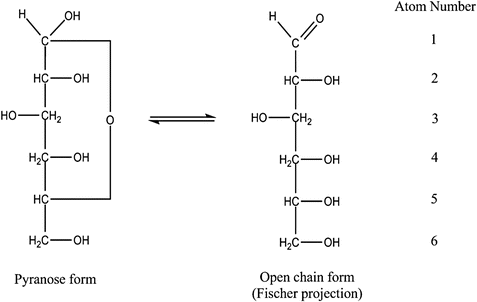
Figure 1.1
Pyranose and open chain forms of D-glucose
Figure 1.2
Structures of D and L glyceraldehyde
Figure 1.3
D and L isomers of glucose
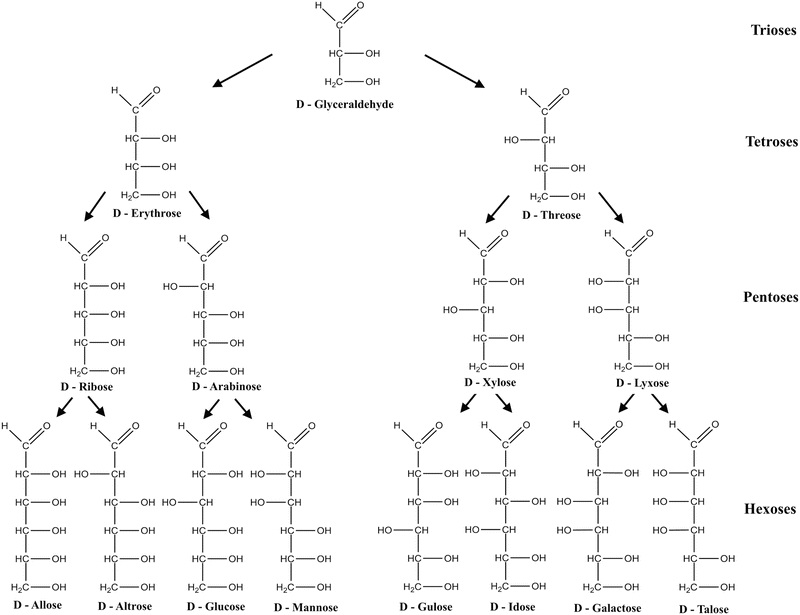
Figure 1.4
Configurational relationship of D-monosaccharides
In solution, glucose molecules continuously change from open chain to ring structure and back again. The formation of the pyranose ring introduces an anomeric carbon in the first position (carbon 1), which allows two different orientations of the hydroxyl group as the ring closes. The two possible forms are designated by a Greek letter prefix as and . When the carbon 1 hydroxyl group is cis- (meaning on the same side) to the hydroxyl group at the carbon 2, the compound is termed -D-glucopyranose or -D-glucose. If the two hydroxyl groups are trans- (meaning on opposite sides) to one another, the compound is -D-glucopyranose or -D-glucose. The structures of - and -D-glucose are shown in the pyranose form and in the cyclic Hayworth configuration in Figure . In the cyclic form, the pyranose ring is considered to be perpendicular to the plane of the paper, with the substituents written to the right below the plane of the ring and those written to the left above the plane of the ring.