Introduction
Good fixation is essential for proper diagnosis. In our department we prefer to receive all breast specimens, except core biopsies, fresh in a plastic bag, immediately after surgical excision whenever this is feasible and in the absence of any suspected infections. The specimen is registered, given a laboratory number, and the pathologist in charge is called to deal with it. For biopsies coming from other hospitals, we ask the surgeons to immerse it in an adequate amount of formalin and send it to us as soon as possible where it is dealt with immediately. If there is going to be a delay, we advise slicing mastectomy specimens before immersing it in formalin. Core biopsies are put in formalin straight after being removed from the patient or, in case of stereotactic biopsies, after x-raying the specimen by the radiologist to ensure the presence of calcification in the cores. The specimen container must be clearly labeled with the patient identity and side and type of specimen. It should be accompanied by a request form detailing the patients name, date of birth, hospital number, the responsible clinician, location, date of request, and relevant clinical history. The information written on the specimen container is checked with that on the request form, particularly as regards the patients name and type and side of the biopsy. Any tissue left after sampling a specimen is retained for 45 weeks after authorization of the report.
Core Biopsies
These are thoroughly dealt with in Chap. but will mention here a few points which we apply in our institution. In our hospital, hard copies of the x-rays of stereotactic core biopsies are not available anymore. Instead, the x-rays are sent to us as e-mail messages from the Radiology Department. They are also available via the hospital net, but we find receiving them as e-mails to be more convenient and easier to access, although the quality of the pictures can be sometimes not as good. Viewing the x-rays when reporting these core biopsies is essential to ensure the presence of the biopsied calcification in the stained sections. If no microcalcification is seen in the three stained sections, or if only fine microcalcification is present in spite of the presence of coarser calcification in the submitted x-ray, further sections have to be cut and examined.
If more than one case is received at the same time, they are not accessioned into the laboratory consecutively but separated by another type of specimen to avoid possible mix-up. The number of cores received from each case is recorded, together with their length and color. In addition to the case number, we usually write the patients name by pencil on the side of the cassette. The specimen is entirely submitted for histological examination, preferably not more than four cores in each cassette. Three shallow levels are cut and stained with hematoxylin and eosin (H&E). Three intervening sections are kept unstained in case they are needed later for the immunohistochemical assessment of hormone receptors and HER2 status if the case proved to be an invasive carcinoma. Additional sections can be requested if more immunohistochemical or special stains are needed. For vacuum biopsies, six levels are cut and stained with H&E if the procedure was carried out for microcalcification. Otherwise, only three levels are cut with intervening spares on coated slides for immunohistochemistry if required.
Dealing with Specimens from Conservative Surgery
Many cases of breast carcinoma, invasive and in situ, are now treated by limited (conservative) surgery rather than mastectomy. These are usually relatively small mono-focal tumors that are detected by palpation or by screening mammography. If the tumor is palpable, it can be removed by a wide local excision or a quadrantectomy depending on the size of the tumor and the size of the breast. If the lesion is impalpable, it is removed by a wire-guided wide local excision. The lesion is localized by inserting a wire radiologically, either by stereotactic or ultrasound technique. The wire guides the surgeon to the site of the tumor, and the area around the tip of the wire is removed.
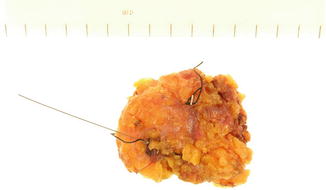
The size of the excised biopsy depends on the size of the tumor as determined radiologically. The surgeon orientates the specimen by attaching sutures of different lengths to the margins of the specimen. At least two margins have to be marked, and these are usually the lateral, indicated by a long suture, and the superior indicated by a short suture (Fig. ). An additional looped suture maybe attached to the anterior surface for confirming the orientation. Metal clips are usually attached to the sutures to help identifying the margins when the specimen is x-rayed (see below).
Fig. 1.1
Specimen with marking sutures
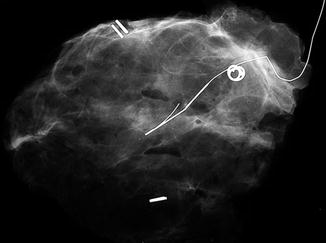
If the lesion was originally detected because of the presence of microcalcification, the excised specimen is usually x-rayed by the surgeon in the operating theater, using a Faxitron, to ensure the removal of all microcalcifications, with a reasonable distance (around 10 mm) between the microcalcification and the margins of the specimen []. These bed biopsies are sent to the pathologist in separate containers labeled with the side from which the biopsy was taken, lateral, medial, etc. and with a stitch attached to one, usually the outer, surface.
Fig. 1.2
Specimen x-ray with attached wire and orientating clips
The role of the pathologist in these cases is not only to make a diagnosis but also to decide whether or not the lesion has been completely excised. If it has not, a re-excision biopsy, or biopsies, will be needed, to ensure the complete removal of the lesion and to minimize the possibility of recurrence.
The specimens received are described, weighed, and measured. To assess completeness of excision, ink has to be applied to the margins of the specimens. For the main specimen, different colored inks are applied to different margins. There are different ways of slicing specimens, and coloring will depend on how the specimen is going to be sliced. As we usually bread slice our specimens, we use four colors, for example, black anterior, blue posterior, red superior, and yellow inferior (Fig. ). A few drops of 1 % acetic acid and absolute alcohol are added to the inked areas to help the adherence of the ink to the specimen surface. A couple of minutes are allowed for the ink to dry, and excess ink is removed by blotting paper. The specimen is then sliced from one margin to the other, say from medial to lateral, into 34 mm thick slices. In this case, the first slice will indicate the medial margin and the last slice the lateral margin; hence there is no need to ink the medial and lateral margins with different colors. This sequence of coloring may vary according to the shape of the specimen. In the above example, if the tumor is felt to be close to the medial or lateral margin, that margin is cut into cruciate sections in order to be able to assess accurately the distance between tumor and margin.