Table of Contents
List of Tables
- Chapter 9
- Chapter 10
List of Illustrations
- Chapter 1
- Chapter 2
- Chapter 3
- Chapter 4
- Chapter 5
- Chapter 6
- Chapter 7
- Chapter 8
- Chapter 9
- Chapter 10
- Chapter 11
- Chapter 12
Guide
Pages
CO2 as a Building Block in Organic Synthesis
Edited by
Shoubhik Das

Editor
Prof. Dr. Shoubhik Das
University of Antwerp Department of Chemistry
Groenenborgarlaan 171
2000 Antwerpen
Belgium
All books published by WileyVCH are carefully produced. Nevertheless, authors, editors, and publisher do not warrant the information contained in these books, including this book, to be free of errors. Readers are advised to keep in mind that statements, data, illustrations, procedural details or other items may inadvertently be inaccurate.
Library of Congress Card No.:
applied for
British Library CataloguinginPublication Data
A catalogue record for this book is available from the British Library.
Bibliographic information published by the Deutsche Nationalbibliothek
The Deutsche Nationalbibliothek lists this publication in the Deutsche Nationalbibliografie; detailed bibliographic data are available on the Internet at .
2020 WILEYVCH GmbH, Boschstr. 12, 69469 Weinheim, Germany
All rights reserved (including those of translation into other languages). No part of this book may be reproduced in any form by photoprinting, microfilm, or any other means nor transmitted or translated into a machine language without written permission from the publishers. Registered names, trademarks, etc. used in this book, even when not specifically marked as such, are not to be considered unprotected by law.
Print ISBN: 9783527346134
ePDF ISBN: 9783527821945
ePub ISBN: 9783527821969
oBook ISBN: 9783527821952
Cover Design Formgeber, Mannheim, Germany
Photochemical and SubstrateDriven CO 2 Conversion
Bart Limburg1, Cristina Maquiln1,2, and Arjan W. Kleij1,3
1Institute of Chemical Research of Catalonia (ICIQ), The Barcelona Institute of Science and Technology (BIST), Av. Pasos Catalans 16, 43007, Tarragona, Spain
2Universitat Rovira i Virgili, Departamentde Qumica Fsica i Inorgnica, Marcell Domingo s/n, 43007, Tarragona, Spain
3Catalan Institute of Research and Advanced Studies (ICREA), Pg. Lluis Companys 23, 08010, Barcelona, Spain
1.1 Introduction
The use of carbon dioxide (CO2) as a raw material in molecular science has been the subject of many investigations. Obviously, the replacement of fossil fuelbased chemistries with those primarily based on CO2 cannot alleviate the challenges we are facing in terms of global carbon emissions and managing the carbon cycle. However, new technologies that can help to partially replace the nonsustainable feedstock into renewable and widely available ones will help to transition to a circular rather than a linear economy [].
The use of CO2 as a reagent in nonreductive coupling reactions (i.e. after integrating the CO2 molecule into an organic substrate, the oxidation state of the carbon center, +4, remains unchanged) has been prominent in the wider area of CO2 catalysis. In this respect, the [3+2] cycloaddition reaction of CO2 and epoxides [].
Recently, various halidefree methodologies have been reported (]. The design of other halidefree systems (with this particular design characteristic although still in its early stage) clearly demonstrates a shift toward the use of more sustainable catalysts in the valorization of CO2 into cyclic carbonates.
What should be the next step in the development of efficient catalysts for cyclic carbonate products (). Apart from the main characteristics of the involved protocols, relevant details encompassing the key mechanistic intermediates will also be highlighted.
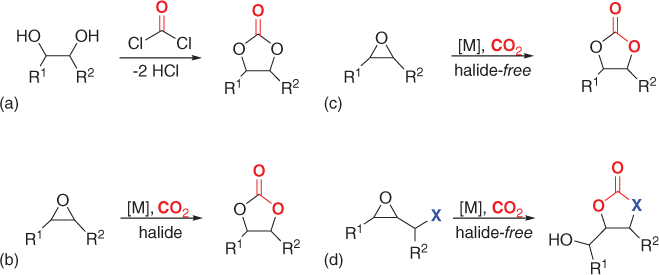
Evolution of the (catalytic) synthesis of cyclic carbonates and future perspective discussed in this chapter. (a) Conventional, (b) contemporary, (c) recent, and (d) current.
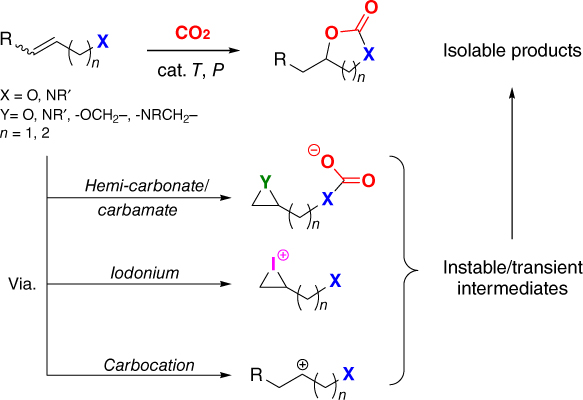
Different approaches to arrive at cyclic carbonates using functional (homo)allylic precursors and various substrateinvolved activation strategies.
1.2 Iodine Activation of (Homo)Allylic Substrates
Molecular iodine (I2) is one of the most widely used iodinating reagents and particularly readily forms reactive iodonium species with alkenes that can afford various addition products in the presence of suitable nucleophiles []. Recently, various research groups have used I2 or other electrophilic Icontaining reagents in reactions that utilize CO2 and comprise of a formal addition of an activated form of CO2 (typically being a carbonate/carbamate intermediate) to I+activated double bonds present within the same substrate.
The first synthesis to use electrophilic, I+directed double bond activation in the formation of either five or sixmembered cyclic carbamates (i.e. oxazolidinones and oxazinones) from CO2 and (homo)allylic amines follows a wellknown strategy that has been previously used for the synthesis of linear and cyclic carbamate derivatives by Yoshida and Saito, respectively [). Both primary and secondary amines were used, but the yields of the heterocyclic products were moderate. Improved yields of the products were achieved by long reaction times and a stoichiometric amount of cesium carbonate.
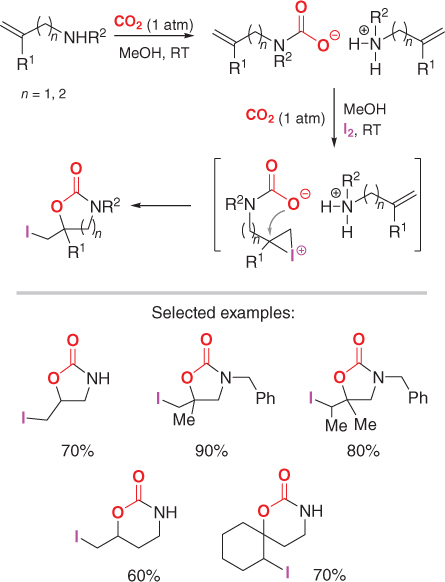
Approach to five and sixmembered heterocycles using CO2 as a reagent and I2 as an olefin activator. Reported yields are those in the presence of Cs2CO3 as an additive.
Later, Muoz and coworkers developed a similar methodology for the synthesis of five and sixmembered cyclic carbamates using (homo)allylic amines, CO2, I2, and a guanidine base ( 2phenyl1,1,3,3tetramethylguanidine , PhTMG ), see ]. However, it was shown that these reactions do not undergo a stereoselective pathway thus forming two possible diastereoisomers without a significant preference for either one. Despite this, if a chiral substituent was used on the amine, the two diastereomers could be separated, resulting in the enantiopure products.
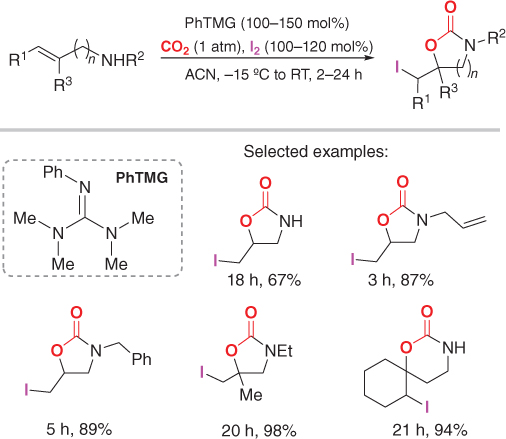
PhTMGmediated formation of five and sixmembered cyclic carbamates.
The methods described thus far use allylic amines for the synthesis of cyclic carbamates with I2 being a key (stoichiometric) reagent to deliver the heterocyclic products. Minakata et al. showed that by changing the iodine source to tertbutyl hypoiodite (tBuOI, a cheap and easily accessible compound obtained in situ from tBuOCl and NaI) as reported by Potter and coworkers []. The specific advantage of using
Next page