Springer International Publishing Switzerland 2015
Jolle Prunet (ed.) Synthesis of Heterocycles by Metathesis Reactions Topics in Heterocyclic Chemistry 10.1007/7081_2015_145
Synthesis of Heteroaromatic Compounds by Alkene and Enyne Metathesis
Nicholas J. Race 1 and John F. Bower 1
(1)
School of Chemistry, University of Bristol, Bristol, BS8 1TS, UK
Abstract
The development of robust and functional group tolerant metathesis catalysts has resulted in a rapid expansion of synthetic methodologies that employ this technology. In this chapter, alkene and enyne metathesis-based methodologies for the de novo construction of heteroaromatic compounds are discussed. These protocols capitalise upon the ability to generate complex olefinic products from the combination of two simpler precursors. Advancement to the heteroaromatic target can then be achieved either directly or after modification of the immediate metathesis product. Strategies that rely upon ring-closing alkene metathesis, ring-closing enyne metathesis and alkene cross-metathesis are outlined, along with applications in target-directed synthesis.
Keywords
Metathesis Heteroaromatic De novo Furan Pyrrole Pyridine Indole
Introduction
Heteroaromatic compounds continue to play an important role in drug discovery, agrochemicals and materials chemistry [].
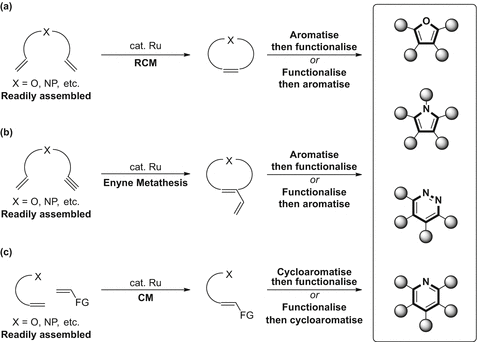
Scheme 1
Metathesis-based strategies to access heteroarenes
Synthesis of Heteroaromatic Compounds by Ring-Closing Alkene Metathesis (RCM)
2.1 Synthesis of 5-Membered Heteroaromatic Compounds by RCM
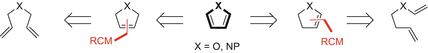
Research in this area has focussed on the use of metathesis for the synthesis of furans, pyrroles and benzofused variants (e.g. indoles). Methodologies for the provision of furans and pyrroles have employed metathesis to form predominantly the C3C4 linkage (Scheme ). Strategies where RCM forms cyclic enol ethers, and therefore the C2C3 linkage of furans, have received less attention, presumably due to the more challenging nature of precursor synthesis. Following the RCM event, aromatisation can be achieved by either (a) oxidation, (b) elimination of a leaving group or (c) isomerisation.
Scheme 2
RCM strategies to access furans and pyrroles
2.1.1 Synthesis of Furans and Pyrroles by RCMOxidation Sequences
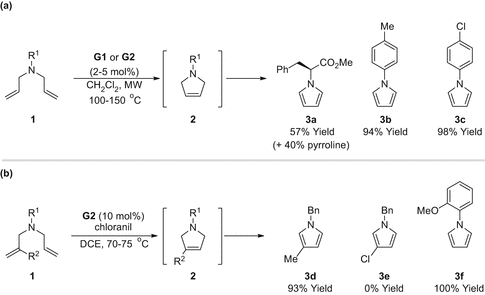
The groups of Xiao and Wilson reported entries to pyrroles wherein RCM of N , N -diallylamines was followed by in situ oxidation of the intermediate pyrroline [] to promote aromatisation.
Scheme 3
RCMoxidation approaches to pyrroles. ( a ) RCMoxidation under oxidant free conditions; ( b ) RCMoxidation using chloranil
Oxidation of dihydrofurans to furans is more challenging than for pyrrolines to pyrroles. In the context of RCM-based furan syntheses, approaches employing NiO2 [].
Scheme 4
RCMoxidation approach to C-2-substituted furans
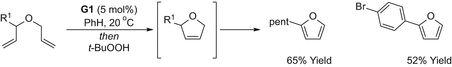
2,5-Disubstituted furans can be accessed via Heck arylation of the corresponding 2,3-dihydrofurans [], was combined with in situ oxidation by chloranil to afford the 2,5-disubstituted furan targets in good yield. A notable feature of this approach is the modification of the Ru-metathesis catalyst such that it can promote a second and mechanistically distinct process.
Scheme 5
RCMisomerisationHeckoxidation approach to 2,5-disubstituted furans
2.1.2 Synthesis of Furans and Pyrroles by RCMElimination Sequences
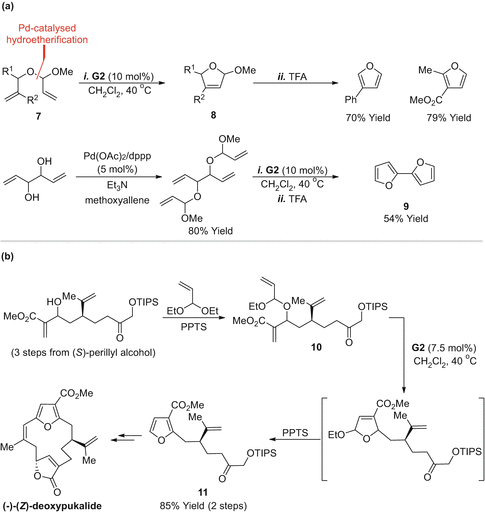
It is well appreciated that many furans and pyrroles are unstable towards oxidants, and this, in turn, limits the scope of the approaches outlined in Sect. ].
Scheme 6
RCMelimination approach to furans and application to (-)-( Z )-deoxypukalide. ( a ) Furans via RCMelimination; ( b ) application to ()-(Z)-deoxypukalide
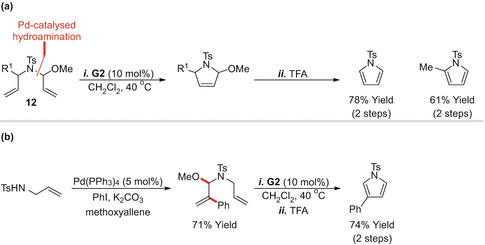
A related approach provides access to pyrrole derivatives [b).
Scheme 7
RCMelimination approaches to pyrroles. ( a ) Hydroamination-RCMelimination approach to pyrroles; ( b ) aminoarylation-RCMelimination approach to pyrroles
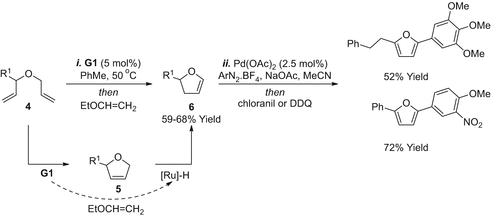
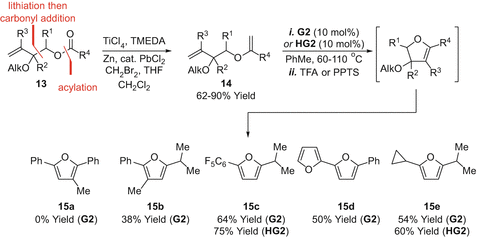
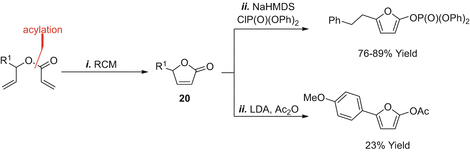
The RCMelimination approaches outlined so far do not provide access to 2,5-disubstituted derivatives, but this can be achieved by altering the position of the leaving group (Scheme ] then provided enol ether metathesis substrates . RCMelimination was then conducted as described earlier to provide the 2,5-disubstituted furan targets 15b - e in moderate to good yield (3875%). In certain cases, the efficiencies of G2 vs HG2 were compared, with the latter providing modestly improved yields. In the case of 15a , ring closure was not achievable, presumably due to the increased steric demands of the methyl-substituted alkene.
Scheme 8
RCMelimination approach to 2,5-disubstituted furans
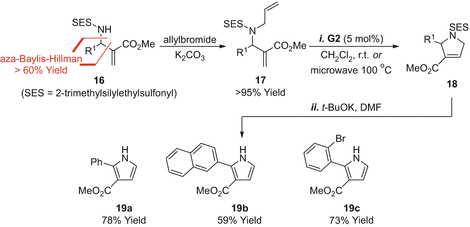
The approach outlined in Scheme ].
Scheme 9
Aza-BaylisHillman-enabled RCM approach to pyrroles
2.1.3 Synthesis of Furans and Pyrroles by RCMIsomerisation Sequences
A final option for achieving aromatisation is to exploit strategies that rely upon isomerisation. A simple access to furans is via the corresponding butenolides, which are formed easily by RCM [].
Scheme 10
RCMenolisation approach to furans
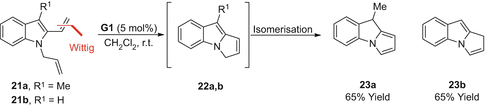
RCMisomerisation approaches to pyrroles are rare (Scheme ]. The initial product 22a underwent isomerisation to afford tricyclic pyrrole derivative 23a in 65% yield. This approach, however, is not general and 22b underwent isomerisation to afford the alternative enamine product 23b in 65% yield.