
Published in 2015 by The Rosen Publishing Group, Inc.
29 East 21st Street, New York, NY 10010
2015 Brown Bear Books Ltd
First Edition
All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted in any form or by any means, without the prior written consent in writing from the publisher.
Library of Congress Cataloging-in-Publication Data Johnson, Rose, 1981
Discoveries in chemistry that changed the world / Rose Johnson.
pages cm. -- (Scientific breakthroughs)
Audience: Grades 5-8.
Includes bibliographical references and index.
ISBN 978-1-4777-8605-5 (library bound)
1. Discoveries in science--History. 2. Chemistry--History. 3. Chemistry--Study and teaching (Elementary) 4. Chemistry--Study and teaching (Middle school) I. Title.
QD40.J625 2015
540--dc23
2014025362
Editor and Text: Rose Johnson
Editorial Director: Lindsey Lowe
Childrens Publisher: Anne ODaly
Design Manager: Keith Davis
Designers: Lynne Lennon and Jeni Child
Picture Researcher: Clare Newman
Picture Manager: Sophie Mortimer
Brown Bear Boo ks has made every attempt to contact the copyright holder. If anyone has any information please contact:
All artwork: Brown Bear Books
Manufactured in the United States of America
Picture Credits
t=top, c=center, b=bottom, l=left, r=right.
FC, R. Gino Santa Maria / Shutterstock, , koya979/ Shutterstock.
Contents
Introduction
Chemistry is the science that studies substances. It tries to figure out what things are made of and how they can be transformed into new substances.
W e may not realize it, but humans have been studying chemistry for thousands of years. Lighting fires, making bread, turning clay into pottery, and purifying metals all involve chemical processes, called reactions.
Simple view
In ancient times, people thought that the world was made up of just four basic ingredients called elements. They were earth, fire, water, and air. It was believed that all natural processes, such as wind, rain, and volcanoes, were driven by these four elements all trying to separate out from each other into layers. The lowest layer was made of cold and heavy earth (and rock). The next layer was water, then air, and at the very top just before you got to the Moonwas a ring of fire.
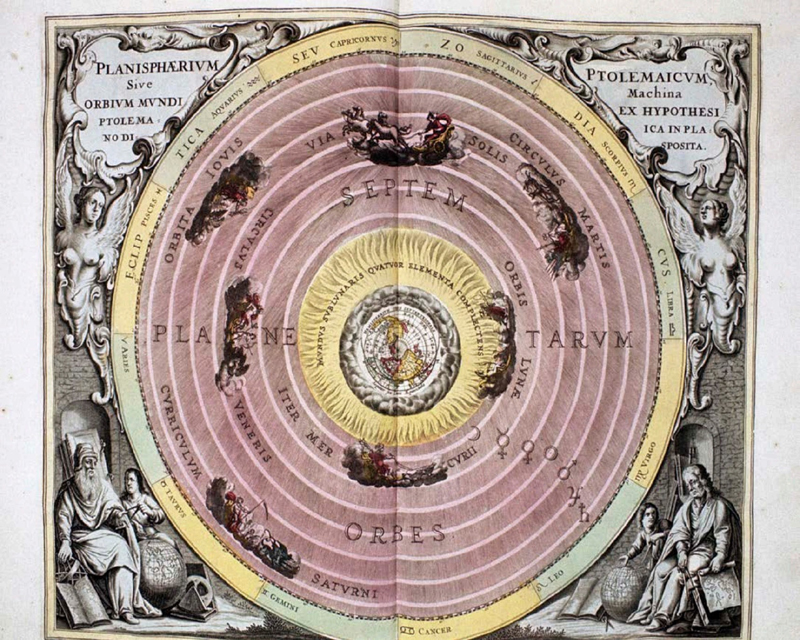
A map of the universe drawn by the ancient Greek philosopher Ptolemy shows rings made up of the different elements.

Purified hot iron pours into a mold. Making iron and other pure metals is part of chemistry.
From wizards to wisdom
The world really is made from elements, and there are 92 natural ones. Many of them were discovered by alchemists. Alchemists were not scientists. They believed that chemical reactions involved magic, and if they could figure it out they could live forever and be fabulously wealthy. We get the word chemistry from alchemist, and chemists still use some of the alchemists techniques. The difference is that chemistry has succeeded in revealing how matter works.

Every substance on Earth is made up of combinations of 92 basic ingredients, such as sulfur (shown below).
Atoms
All the substances we find on Earth are made up of building blocks called atoms. This is actually a very ancient idea, first thought up by Greek philosophers.

About 2,400 years ago, Democritus came up with a theory of atoms that fits quite closely with our modern understanding.
T he idea of atoms was developed by two philosophers, Leucippus of Miletus (400s BCE) and his student, Democritus (c460370 BCE), who lived in Turkey in the 5th century BCE. Their idea for atoms came from the suggestion that the world was in fact an illusion.
Chasing a tortoise
Another Greek called Xeno told the story of Achilles (a great warrior) chasing a tortoise. Every time Achilles halved the distance between himself and the tortoise, the reptile had crawled on a little bit. So, said Xeno, Achilles always got closer but could never catch up.

According to the Greek philosophers, if the world did not have atoms, a tortoise could outrun a humanor at least appear to.
Indivisible units
Xeno used his story to suggest that motion was actually impossible. Every distance had to be covered in an infinite number of stepsyou would travel forever and never get anywhere. In response Leucippus suggested that the universe was made from tiny units that were the smallest size possibleso small they could not be halved. He called them atoms, which means indivisible.
Properties of matter
Democritus used this idea of atoms to describe the properties of natural substances: The atoms of water were slippery, fire atoms were spiky, while earth was made from sticky atoms that clung together. Although atoms are more complicated than this, centuries later, scientists found that everything is made from atoms.

In the days of Democritus, it was thought everything was made of just four elements: air, fire, water and earth.
FACTS
Democritus is known as the Laughing Philosopher He thought the universe had no purpose and so it was best to just be happy.
There are 90 types of atoms that occur naturally on Earth and several more that are made in laboratories.
Discovering Elements
The basic substances studied by chemistry are the elements. Scientists have discovered 118 of them so far. The first recorded discovery was in 1669.
IMPLICATIONS
Phosphorus is very useful. It is added to the fertilizers that help crops grow. It is also one of the chemicals used in matches, which make it possible to create fire anywhere.
A n element is a substance that cannot be broken into any simpler ingredients. Ancient people believed the Universe was made from just four or five elements, but by the 17th century it was understood there were actually many more. Substances like carbon, gold, iron, copper, and sulfur were being classed as elements as well.
Getting rich quick
The people who studied elements at this time were called alchemists. They were more like wizards than scientists. They were searching for magical substances that could make them live forever and turn any substance into gold.

Rubbing these matches on a rough surface makes phosphorus in the head burst into flames.

Next page





















