1. The Cerebral Circulation
As in the case of each regional circulation, the cerebral circulation has special characteristics and unparalleled control mechanisms. The brain also holds a particular role, as it lodges the principal centers of the cardiovascular and respiratory control system of the body. The brain and the spinal cord are enclosed in rigid osseous and dural structures. As envisaged centuries earlier by the Monro-Kellie concept, the volume of the intracranial contents cannot change [], and consequently, a physiologic balance must be maintained between the cerebral parenchyma, cerebrospinal fluid, and blood. The cerebral parenchyma is constituted by nerve cells, nerve fibers, neuroglia, intercellular fluid, and vascular tissue all enclosed by the meninges. In a simplified scheme, the balance must be maintained between the cerebral parenchyma, cerebrospinal fluid, and the arterial and venous compartments, respectively. Reciprocal volume changes naturally occur, for instance, under normal circumstances, arteriolar dilatation is made possible by a reduction of the venous volume without increasing the resistance to flow. On the other hand, disproportionate vasodilation and subsequent vasogenic edema will increase intracranial pressure generating severe neurologic complications and eventually death.
The brain weighs about 2% of the body mass but has a high metabolic rate and receives between 15 and 20% of the total cardiac output and uses 18% of the oxygen absorbed by the lungs; the resting blood flow and O2 consumption per unit of mass, of the central nervous system (CNS), are surpassed only by that of the kidneys and myocardium. The cerebral arteriovenous difference is high, being exceeded only by the myocardium and skeletal muscle [].
Under resting conditions, the cerebrovascular resistance is moderate with the contribution of the large arteries accounting for almost half of the total vascular impedance. However, among the numerous vasomotor stimuli, only a few have constrictor effect; an active contraction of the walls of the vessels of the cerebral circulation is never a strong response, and the tonic sympathetic vasoconstrictor discharge does not characterize the cerebral circulation. The basal state of the cerebral vessels is the result of a combination of factors that produce vasodilatation.
Two major arterial systems contribute to the vascularization of the brain: the carotid and the vertebrobasilar systems. Both belong to the superior aortic system and earn a particular organization and distribution of flow from the very early stages of development with a preferential delivery of oxygenated blood to the brain (the same physiologic adaptation is evident in diving species, including reptiles, chelonians, and crocodilians). Some important features of the arteries vascularizing the brain are as follows. Anastomoses are numerous and multidirectional in the superior aortic system and are constituted between the internal and external carotid, between the carotid and vertebral arteries, between the subclavian and vertebral or external carotid arteries, and between the right and left sides. Venous drainage has similar characteristics, and an important amount of mixing of arterial or venous blood is present under normal conditions or in various disease states. There is an apparent symmetry of the vessels of the brain although unparalleled structures render the right/left division less evident, concomitantly compensating any obstacle or impediment to arterial or venous flow: the basilar trunk, the arterial circle of Willis, the anterior communicating artery, the superior sagittal sinus, the cavernous sinus, and the pterygoid plexus. There is also a wide range of individual variations regarding the ratio between the amount of blood carried by the carotid and the vertebrobasilar system, the individual differences between the right and left vertebral arteries, the difference in the diameter of the internal jugular veins, the inverse relationship between the internal and external jugular veins, and so forth. Numerous anatomical variations in the origin, course, and termination of the cerebral vessels are also encountered including the presence of vestigial vertebro-carotid anastomoses. As a result, the picture of the cerebral vascularization is protean, and the response to the various physiologic or pathologic conditions is varied.
The branches of the cerebral arteries eventually give off pial arteries vessels on the very surface of the brain lodged within the pia-arachnoid and surrounded by cerebrospinal fluid. These arteries give rise to smaller penetrating arteries, contained within the Virchow-Robin spaces (continuation of the subarachnoid space) and which eventually become parenchymal arterioles once they penetrate the brain tissue; the latter are sheathed by the astrocytic end feet which contribute to the blood-brain barrier. Astrocytes play a major role in capillary function as they regulate the cerebral blood flow, the ion, and water hemostasis. The penetrating and the parenchymal arteries are terminal vessels [].
The cerebral arteries and arterioles have a well-developed internal elastic membrane but no external elastic membrane; there are also fewer elastic elements in the tunica media. Due to the lack of external elastic membrane, the intracerebral arteries are more prone to rupture. The smooth muscle cells in the tunica media are circularly oriented with a zero-degree obliquity. A similar disposition is present in the larger pial veins. The cerebral veins are devoid of valves. The intracerebral veins are very thin walled.
The density of the intracerebral capillaries is high, as practically every neuron has its own capillary. Various conditions may alter capillary density: chronic hypoxia increases capillary density, while hypertension causes a rarefaction of the vessels. A neurovascular unit (see below) is composed of endothelial cells, vascular mural cells (vascular smooth muscle and pericytes), glial cells (astrocytes and microglia), and neurons. In the intracerebral arteries, vascular smooth muscle cells occupy most of the vascular wall thickness. At the level of brain capillaries, pericytes replace smooth muscle cells and are attached to the vascular basement membrane. Pericytes extend multiple cytoplasmic processes which encircle the endothelial cells; the pericyte/endothelial cell ratio is high in the brain as compared to other organs and tissues [].
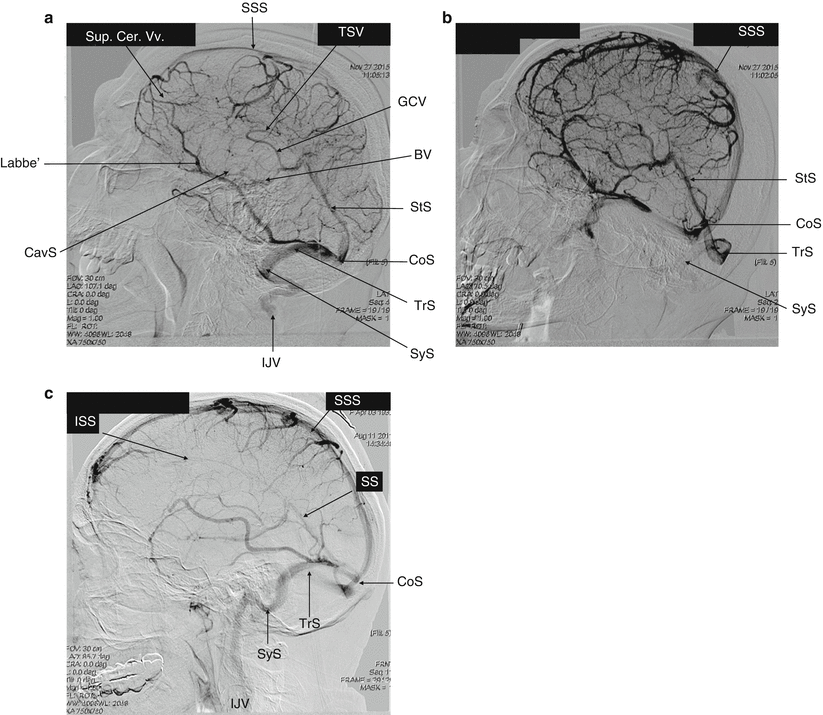
The veins draining the cerebral hemispheres can be classified in Fig.. The superficial cortical veins drain the cortex and subcortical white matter. The deep or central veins (the subependymal veins, internal cerebral veins, the basal vein, and the great cerebral vein of Galen) drain the deep white matter and gray matter surrounding the lateral and third ventricles and the basal cistern. The cortical veins empty into the superior sagittal sinus. Both venous systems (superficial and deep) drain blood toward the confluence of sinuses, the sigmoid sinuses, and eventually, the internal jugular veins. The cerebellum is drained by the inferior cerebellar veins and occipital sinuses. The veins draining the brainstem terminate in the inferior and transverse petrosal sinuses.