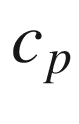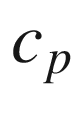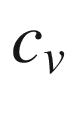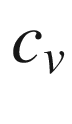Mechanical Engineering Series
Series Editor
Francis A. Kulacki
Department of Mechanical Engineering, University of Minnesota, Minneapolis, MN, USA
The Mechanical Engineering Series presents advanced level treatment of topics on the cutting edge of mechanical engineering. Designed for use by students, researchers and practicing engineers, the series presents modern developments in mechanical engineering and its innovative applications in applied mechanics, bioengineering, dynamic systems and control, energy, energy conversion and energy systems, fluid mechanics and fluid machinery, heat and mass transfer, manufacturing science and technology, mechanical design, mechanics of materials, micro- and nano-science technology, thermal physics, tribology, and vibration and acoustics. The series features graduate-level texts, professional books, and research monographs in key engineering science concentrations.
More information about this series at http://www.springer.com/series/1161
Lin-Shu Wang
A Treatise of Heat and Energy
Lin-Shu Wang
Stony Brook University, Stony Brook, NY, USA
ISSN 0941-5122 e-ISSN 2192-063X
Mechanical Engineering Series
ISBN 978-3-030-05745-9 e-ISBN 978-3-030-05746-6
https://doi.org/10.1007/978-3-030-05746-6
Springer Nature Switzerland AG 2020
This work is subject to copyright. All rights are reserved by the Publisher, whether the whole or part of the material is concerned, specifically the rights of translation, reprinting, reuse of illustrations, recitation, broadcasting, reproduction on microfilms or in any other physical way, and transmission or information storage and retrieval, electronic adaptation, computer software, or by similar or dissimilar methodology now known or hereafter developed.
The use of general descriptive names, registered names, trademarks, service marks, etc. in this publication does not imply, even in the absence of a specific statement, that such names are exempt from the relevant protective laws and regulations and therefore free for general use.
The publisher, the authors and the editors are safe to assume that the advice and information in this book are believed to be true and accurate at the date of publication. Neither the publisher nor the authors or the editors give a warranty, expressed or implied, with respect to the material contained herein or for any errors or omissions that may have been made. The publisher remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This Springer imprint is published by the registered company Springer Nature Switzerland AG
The registered company address is: Gewerbestrasse 11, 6330 Cham, Switzerland
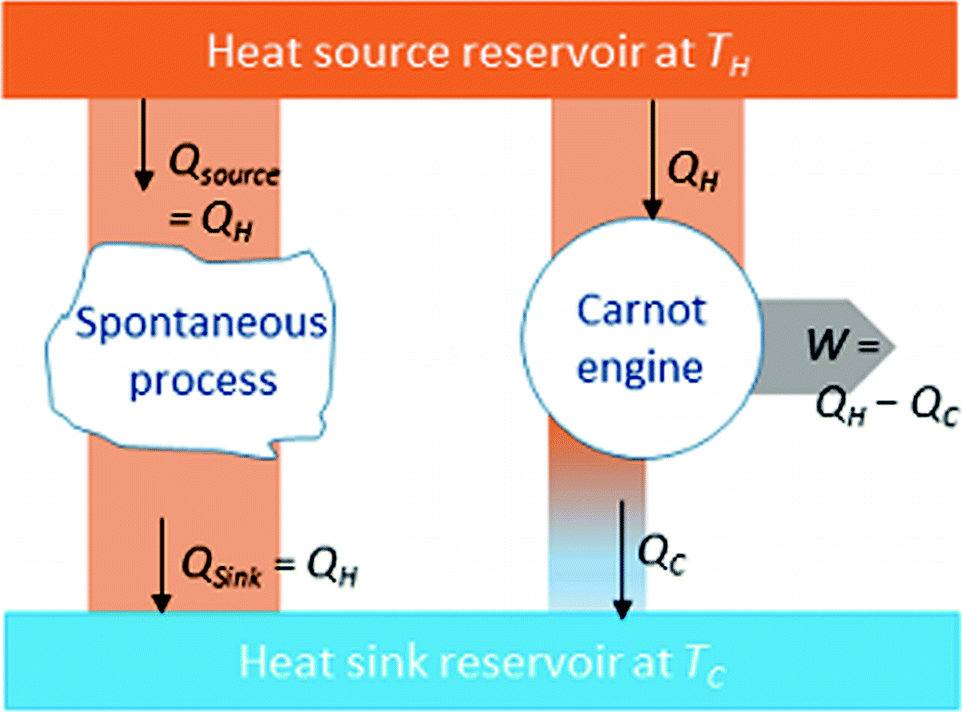
Heat and work flow for a Carnot cycle, which is an example of extracting from the heat sink reservoir heat of the amount, 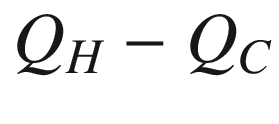 , (that would have been lost in a spontaneous heat transfer process) for the production of work,W=
, (that would have been lost in a spontaneous heat transfer process) for the production of work,W= 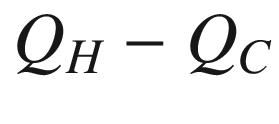 .
.
To Ming
Preface
Thermodynamic understanding of heat and energy is based on the mechanical theory of heat (MTH), which resulted from the synthesis, by Kelvin and Clausius, of Carnots theory of heat and the MayerJoule principle. Yet, there are no good definitions for heat or energy in the current literature on thermodynamics. It is noted that the advent of the entropy principle created the scientific stream of thermodynamics (a new stream branched off from its original source, the engineering stream) and led to, in quick succession, the successful formulation of equilibrium thermodynamics. Here, I make the case that the impression of the KelvinClausius synthesis success is formed from its success in producing a coherent system of equilibrium thermodynamics, not in resulting in a coherent system of engineering stream of thermodynamicsthe failure of which is reflected in the fact that engineering thermodynamics cannot even talk about heat and energy without self-contradictions as well as fail to provide students of thermodynamics real grasp on reversibility. This disquisitionessay makes the case that the uneven achievement of Joule, Kelvin, and Clausius is because they made the classic error of equating correlation between heat and work to causality between heat and work, and, as a result, prevented the (later) formulation of the entropy principle from realizing its full power. While this error has been pointed out in a number of papers, the authors of those papers advocated, for removing the error, a return to Carnots theory as a caloric theory of heat. That was clearly a mistake: it is argued here that Carnots theory is a relational theory of heat not an ontological theory and, in fact, it can be made to incorporate with, ontologically, either the caloric theory or MTH. This disquisition essay presents a relational, i.e., predicative, theory of heat embracing fully MTHs ontology for an updated understanding of heat, spontaneous energy conversion, and reversible-like processes.
Lin-Shu Wang
Stony Brook, USA
Symbols and Abbreviations
AArea, 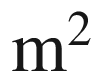
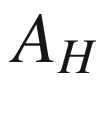
Helmholtz function, 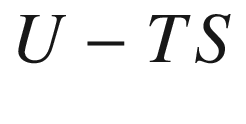 , kJ
, kJ
AF
Airfuel ratio
cSpeed of sound, m/s
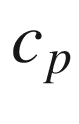
Constant pressure specific heat, kJ/kg-K
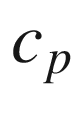
Constant pressure molar specific heat, kJ/kmol-K
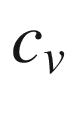
Constant volume specific heat, kJ/kg-K
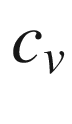
Constant volume molar specific heat, kJ/kmol-K














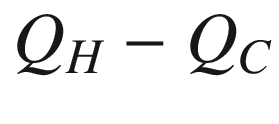 , (that would have been lost in a spontaneous heat transfer process) for the production of work,W=
, (that would have been lost in a spontaneous heat transfer process) for the production of work,W= 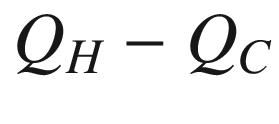 .
.
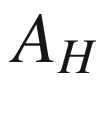
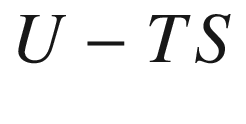 , kJ
, kJ