Yulong Ding (editor) - Thermal energy storage : materials, devices, systems and applications
Here you can read online Yulong Ding (editor) - Thermal energy storage : materials, devices, systems and applications full text of the book (entire story) in english for free. Download pdf and epub, get meaning, cover and reviews about this ebook. year: 2021, genre: Romance novel. Description of the work, (preface) as well as reviews are available. Best literature library LitArk.com created for fans of good reading and offers a wide selection of genres:
Romance novel
Science fiction
Adventure
Detective
Science
History
Home and family
Prose
Art
Politics
Computer
Non-fiction
Religion
Business
Children
Humor
Choose a favorite category and find really read worthwhile books. Enjoy immersion in the world of imagination, feel the emotions of the characters or learn something new for yourself, make an fascinating discovery.
- Book:Thermal energy storage : materials, devices, systems and applications
- Author:
- Genre:
- Year:2021
- Rating:4 / 5
- Favourites:Add to favourites
- Your mark:
- 80
- 1
- 2
- 3
- 4
- 5
Thermal energy storage : materials, devices, systems and applications: summary, description and annotation
We offer to read an annotation, description, summary or preface (depends on what the author of the book "Thermal energy storage : materials, devices, systems and applications" wrote himself). If you haven't found the necessary information about the book — write in the comments, we will try to find it.
Thermal energy storage : materials, devices, systems and applications — read online for free the complete book (whole text) full work
Below is the text of the book, divided by pages. System saving the place of the last page read, allows you to conveniently read the book "Thermal energy storage : materials, devices, systems and applications" online for free, without having to search again every time where you left off. Put a bookmark, and you can go to the page where you finished reading at any time.
Font size:
Interval:
Bookmark:
Chapter 1
Thermodynamics for Thermal Energy Storage
Yulong Ding
a Birmingham Centre for Energy Storage & School of Chemical Engineering, University of BirminghamEdgbastonBirmingham B15 2TTUK
Thermal energy storage processes involve the storage of energy in one or more forms of internal, kinetic, potential and chemical; transformation between these energy forms; and transfer of energy. Thermodynamics is a science that deals with storage, transformation and transfer of energy and is therefore fundamental to thermal energy storage. Thermodynamics can be categorised into classical thermodynamics, statistical mechanics, chemical thermodynamics, equilibrium thermodynamics and non-equilibrium thermodynamics. This chapter introduces classical thermodynamics concepts and laws although they are applicable to other categories. An attempt is made to relate these to thermal energy storage where appropriate.
Thermodynamics is a science that deals with storage, transformation and transfer of energy. It is fundamental to the topics of thermal energy storage, which consists of a collection of technologies that store thermal (heat or cold) energy and use the stored energy directly or indirectly through energy-conversion processes when needed. Thermodynamics can be categorised into classical thermodynamics, statistical thermodynamics, chemical thermodynamics, equilibrium thermodynamics and non-equilibrium thermodynamics. This chapter introduces the classical thermodynamics concepts and laws considered to be most relevant to thermal energy storage. Attempts are made to relate these to thermal energy storage where appropriate.
Thermodynamics is a scientific discipline born in the 19th century to describe the operation of steam engines, which enabled the first industrial revolution that started in the UK and then spread around the world. It can be categorised into the following fundamentally related branches:
- Classical thermodynamics, developed in the 19th century, describes the states of a thermodynamic system at equilibrium using macroscopically measurable properties.
- Statistical thermodynamics, emerged in the late-19th century and early-20th century with the development of atomic and molecular theories, supplements the classical thermodynamics with an interpretation of the microscopic interactions and quantum-mechanical states.
- Chemical thermodynamics concerns the interrelation of energy with chemical reactions or with a physical change of state within the confines of the laws of thermodynamics.
- Equilibrium thermodynamics examines the transfer of matter and energy in a system or a body that, by its surroundings, can be driven from one state of thermodynamic equilibrium to another.
- Non-equilibrium thermodynamics deals with systems that are not in thermodynamic equilibrium.
A thermal dynamic system is a device or combination of devices (e.g., for energy storage) that contain a certain quantity of matter (e.g., thermal energy storage materials). Anything outside the system is termed surroundings. The whole universe is made of the system and the surroundings. Systems have no mass exchange but can have energy exchange with the surroundings, and hence are termed closed systems or controlled-mass systems in some of the literature. A system with no energy exchange with surroundings is called an isolated system. A well thermally insulated thermal energy storage system can be regarded as an isolated system during its storage period.
Control volume refers to a volume in space into which, or from which, a substance flows. The control volume is also called an open system in some books. A thermal energy storage system can be regarded as a control volume or an open system during charge and discharge processes if the storage material also acts as a heat transfer fluid.
A phase refers to a quantity of matter that is homogeneous throughout. There are three phases in nature: gas, liquid and solid. A mixture of different phases is heterogeneous with a distinct macroscopic boundary between the phases. A sensible thermal energy storage material often exists as a single phase, whereas a latent heat storage material can be a single-phase (before or after phase change) or a two-phase mixture (during phase change).
A property is any quantity that serves to describe a system. Examples of thermodynamic properties are temperature and pressure. The state of a system refers to its condition as described by giving values to its properties at a particular instant. The features of thermodynamic properties are that they have unique values when the system is in a particular state, and the value does not depend on previous states that system has passed through; they are not a function of path and are therefore an exact differential:
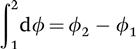 1.1
1.1
where is a property; 1 is the property value at State 1 and 2 is the property value at State 2. There are two types of properties, intensive and extensive:
- Intensive properties do not depend on the mass of the system. Examples include temperature, pressure, specific volume and density.
- Extensive properties do depend on mass of the system. Mass and volume are examples of extensive properties.
The features of thermodynamic properties provide the basis for the development of methods for the calculation of important parameters such as energy-storage capacity, energy density and state-of-charge of thermal energy storage systems based on the property values during charge and discharge processes.
Thermodynamic equilibrium is the state of a system in which it is in mechanical, chemical and thermal equilibrium with no tendency for spontaneous change. This implies that all properties of a system at equilibrium have no tendency of change with time. A thermodynamic process refers to a path of successive states through which a system passes, whereas a quasi-equilibrium process refers to a process in which a system passing from one state to another deviates infinitesimally from equilibrium. A cycle is the process when a system experiences a series of quasi-equilibrium changes and returns to its initial state. A thermodynamic process is called isothermal, isobaric or isometric (or isochoric) if the process has a constant temperature, pressure or volume, respectively.
A typical thermal energy storage system is often operated in three steps: (1) charge when energy is in excess (and cheap), (2) storage when energy is stored with no demand and (3) discharge when energy is needed (and expensive). These three steps are called a process, and the three steps the system undergoes form a cycle if the state of the system returns to its initial state. Most of the sensible heat storage processes, particularly those using solid materials, can be regarded as isobaric. Due to thermal expansion, the majority thermal energy storage processes are non-isometric. Isothermal processes occur during the phase change of latent heat storage systems and the storage step.
Thermal energy storage processes often involve changes in temperature, volume and/or pressure. The relationship between these properties is therefore important for the design and operation of thermal energy storage systems. This subsection briefly discusses the pressure-volume-temperature (PVT) behaviour. The focus is on pure substances that have a homogeneous and invariable chemical composition and although they may exist in more than one phase, the chemical composition is the same in all phases. For example, a mixture of water, ice and steam is a pure substance, while a mixture of liquid air and gaseous air is not a pure substance. Sometimes, a mixture of gases, such as air, could be considered as a pure substance as long as there is no change of phase.
Next pageFont size:
Interval:
Bookmark:
Similar books «Thermal energy storage : materials, devices, systems and applications»
Look at similar books to Thermal energy storage : materials, devices, systems and applications. We have selected literature similar in name and meaning in the hope of providing readers with more options to find new, interesting, not yet read works.
Discussion, reviews of the book Thermal energy storage : materials, devices, systems and applications and just readers' own opinions. Leave your comments, write what you think about the work, its meaning or the main characters. Specify what exactly you liked and what you didn't like, and why you think so.









