Introduction
Proteins are already used for more than 100 years to treat or prevent diseases in humans. It started in the early 1890s with serum therapy for the treatment of diphtheria and tetanus by Emile von Behring and others. The antiserum was obtained from immunized rabbits and horses. Behring received the Nobel Prize for Medicine in 1901 for this pioneering work on passive immunization. A next big step in the development of therapeutic proteins was the use of purified insulin isolated from pig or cow pancreas for the treatment of diabetes type I in the early 1920s by Banting and Best (in 1923 Banting received the Nobel Prize for this work). Soon after the discovery of insulin, the pharmaceutical company Eli Lilly started large-scale production of the pancreatic extracts for the treatment of diabetes. Within 3 years after the start of the experiments by Banting, already enough animal-derived insulin was produced to supply the entire North American continent. Compare this to the present average time-to-market of a new drug (from discovery to approval) of 13.5 years (Paul et al. ).
Thanks to advances in biotechnology (e.g., recombinant DNA technology, hybridoma technology), we have moved almost entirely away from animal-derived proteins to proteins with the complete human amino acid sequence.
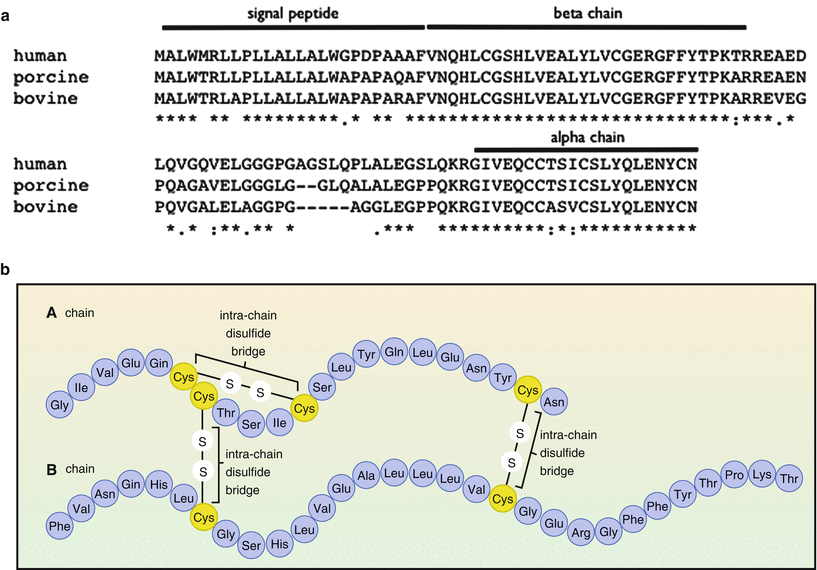
Such therapeutic human proteins are less likely to cause side effects and to elicit immune responses. Banting and Best were very lucky. They had no idea about possible sequence or structural differences between human and porcine/bovine insulin. Nowadays, we know that porcine insulin differs only with one amino acid from the human sequence and bovine insulin differs by three amino acids (see Fig. ). Since then a large number of biopharmaceuticals have been developed. There are now almost 200 human proteins marketed for a wide range of therapeutic areas.
Figure 1.1
( a ) Multiple alignment ( http://www.ebi.ac.uk/Tools/msa/clustalw2 ) of the amino acid sequences of human, porcine, and bovine preproinsulin. : identical residue. ( b ) Schematic drawing of the structure of insulin. The alpha and beta chain are linked by two disulphide bridges. Both the one-letter and three-letter codes for the amino acids are used in this figure: alanine ( ala , A), arginine ( arg , R), asparagine ( asn , N), aspartic acid ( asp , D), cysteine ( cys , C), glutamic acid ( glu , E), glutamine ( gln , Q), glycine ( gly , H), histidine ( his , H), isoleucine ( ile , I), leucine ( leu , L), lysine ( Lys , K), methionine ( met , M), phenylalanine ( phe , F), proline ( pro , P), serine ( ser , S), threonine ( thr , T), tryptophan ( trp ,W), tyrosine ( tyr , Y), and valine ( val , V) (Figure b is taken from Wikipedia).
Pharmaceutical Biotechnology, Why This Book, Why This Chapter?
In this book we define pharmaceutical biotechnology as all technologies needed to produce biopharmaceuticals (other than (nongenetically modified) animal- or human blood-derived medicines). Attention is paid both to these technologies and the products thereof. Biotechnology makes use of findings from various research areas, such as molecular biology, biochemistry, cell biology, genetics, bioinformatics, microbiology, bioprocess engineering, and separation technologies. Progress in these fields has been and will remain a major driver for the development of new biopharmaceuticals. Biopharmaceuticals form a fast-growing segment in the world of medicines opening new therapeutic options for patients with severe diseases. This success is also reflected by the fast growth in global sales. Double-digit growth numbers were reported over the last 25 years, reaching $80 billion in 2012. Five drugs in the top ten of drugs with the highest sales are biopharmaceuticals (2010), clearly showing the therapeutic and economic importance of this class of drugs.
Until now biopharmaceuticals are primarily proteins, but therapeutic DNA or RNA molecules (think about gene therapy products, DNA vaccines, and RNA interference-based products; , respectively) may soon become part of our therapeutic arsenal.
Therapeutic proteins differ in many aspects from classical, small molecule drugs. They differ in size, composition, production, purification, contaminations, side effects, stability, formulation, regulatory aspects, etc. These fundamental differences justify paying attention to therapeutic proteins as a family of medicines, with many general properties different from small molecules. These general aspects are discussed in the first set of chapters of this book (General Topics). After those general topics, the different families of biopharmaceuticals are dealt with in detail. This first chapter should be seen as a chapter where many of the basic elements of the selection, design, and production of biopharmaceuticals are touched upon. For in detail information the reader is referred to relevant literature and other chapters in this book.
Economics and Use
Newly introduced biopharmaceuticals are very expensive. This is partly due to the high development cost (~$1.5 billion), but this is not different from the development costs of small molecule drugs (Paul et al..
As mentioned above, the number of patients for many marketed therapeutic proteins is relatively small. This has several reasons. The high price of therapeutic proteins makes that they are used primarily for the treatment of the relative severe cases. The specificity of many therapeutic proteins makes that they are only effective in subgroups of patients (personalized medicine). This is in particular true for the monoclonal antibodies used to treat cancer patients. For instance, the antibody trastuzumab (Herceptin) is only approved for breast cancer patients with high expression levels of the HER2 receptor on the tumor cells (20 % of breast cancer cases). Other examples from the cancer field are the monoclonal antibodies cetuximab and panitumumab for the treatment of metastatic colorectal cancer. Both antibodies target the EGF receptor. Successful treatment of a patient with one of these monoclonal antibodies depends on (1) the presence of the EGF receptor on the tumor and (2) the absence of mutations in signaling proteins downstream of the EGF receptor (KRAS and BRAF). Mutations in downstream signaling proteins cause the tumor to grow independently from the EGF receptor and make the tumor nonresponsive to the antagonistic monoclonal antibodies.