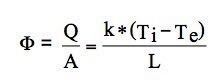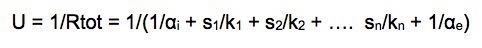Davide Lanzoni
BUILDING THERMOGRAPHY
(including blower door and heat flux meter)
BUILDING THERMOGRAPHY
(including blower door and heat flux meter)
Copyright 2014 Davide Lanzoni
e-book creation and cover design: www.iltuoebook.it
Table of contents
1. Foundations of Thermography
For an offer for purchasing a thermal imager, a blower door, other instrumentation, software or thermography training courses, you may fill our form at link:
http://www.saige.it/e-book_en.aspx
1.1 Heat and temperature
Heat is a form of energy whose main property is to move from a hotter body to another material body that is cooler, where material body is meant as a certain amount of any substance, whether solid, liquid or gaseous.
Heat is a concept that is different from temperature, but nevertheless connected to it. Heat is in fact an intangible magnitude and is not directly measurable; what can be measured are the effects of heat that can be attributed to variations in temperature. When we talk about heat, in reality what is being referred to is a flow of heat (and the amount of thermal energy that passes through a surface per time unit): the sensation of heat or cold that we feel when we touch an object depends on the direction of heat flow (if it flows from or towards our hand respectively). Heat moves spontaneously from a hotter object (or from a medium) to a colder one. When the two media reach the same temperature, thermal equilibrium is reached in the system and heat ceases to flow.
When referring to temperature, what is meant is the extent of the degree of molecular agitation. The temperature of the material depends on its physical characteristics. These include heat capacity and specific heat which vary depending on the phase (gas, liquid, solid) in which the material is found.
The principles of thermodynamics can be formulated as follows:
1. Zero principle: if a body "A" is in thermal equilibrium with a body "B" and "B" is in thermal equilibrium with a body "C", then "A" and "C" are in equilibrium with each other.
2. First principle: the variation of the internal energy of a closed thermodynamic system is equal to the difference between the heat supplied to the system and the work performed by the system on the environment. The corresponding mathematical formulation is expressed as: U = Q - L, where U is theinternal energy of the system, Q is the heat supplied to the system and L the work performed by the system.
3. Second principle: there are different statements, all equivalent, and each of the formulations highlights a particular aspect. It states that it is impossible to create a cyclic machine whose only result is the transfer of heat from a cold body to a hot one (statement of Clausius) or, equivalently, that it is impossible to achieve a transformation whose result is not only to convert into mechanical work the heat taken from a single source (statement of Kelvin). The latter limitation denies the possibility of producing so-called perpetual motion of the second kind.
4. Third principle: it is closely related to the second, and in some cases it is seen as a consequence of the latter. It can be stated by saying that it is impossible to reach absolute zero with a finite number of transformations.
Energy, from the molecular vibration of the material, is transformed into heat when it is released (the internal energy of coal, for example, is released when it is burned) or when there are conditions such that the energy is transferred from the medium in question to another at a lower temperature (second principle of thermodynamics). This transfer is measured by thermometers that return a temperature value. The greater the internal energy, the greater the temperature will be.
Once the heat leaves the object, this is definitively lost: the heat may be acquired by a heat source at a higher temperature. To maintain a flow of heat in a stationary condition, a constant temperature difference must be ensured.
Temperature is expressed in both absolute and relative terms. The two absolute scales are that of Rankine ( R, in the Anglo-Saxon metric system) and Kelvin (K, in the international metric system); the relative corresponding scales are those that express degrees Fahrenheit (F) and Celsius (C). The Fahrenheit scale places the melting point of ice at 32F and the boiling point of water at 212F, with 180 divisions. Absolute zero on the international scale corresponds to -273.15 C or 0 K.
Conversions between temperature scales are:
t (C) = [t(F) 32] x 5/9
t (F) = [t (C) x 9/5] + 32
T (K) = t (C) + 273,15
1.2 Heat transmission
Heat is transmitted by conduction, convection, and radiation.
1.2.1 Transmission of heat by conduction
It has been mentioned that conduction occurs between media at different temperature, in direct contact with each other. The ability of a medium to convey heat is determined by its conductivity.
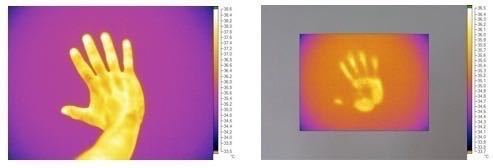
Fig.1.1: heat conduction from a hand to a wall
The conductivity of a material affects in a manner that is directly proportional the quantity of heat that is able to flow from the medium: the greater the conductivity, the lower the thermal resistance. Metals have a very high thermal conductivity: this is because the atoms that compose them are very mobile: heat excites the atoms of the material that start to vibrate, colliding with the surrounding atoms to which is transferred a part of their energy. In this way energy is passed from atom to atom by the material at a higher temperature towards the one at a lower temperature.
Thermal conduction follows Fourier's Law :
where:
Q/A and the flow of heat [W/m2]
K (or ) is the thermal conductivity [W/(mK)]
L is the thickness of the material with respect to the direction of heat [m]
Ti and Te are the internal and external temperature [C or K] if, for example, the hot and cold body are the inside and outside of the building.
The medium is defined as good conductor when k is high. Air is a good insulator while water is not: thus, if the insulating material that covers a wall is soaked with water, its conductivity increases, increasing the heat losses of the building. The best thermal conductors are metals.
The length L of the path of heat in the material also influences the flow of heat: it would not therefore be sufficient to have a material with a low k, but also to have consistent thickness: the dispersion of heat will be smaller, the greater the thickness of the wall. In summary:
The ratio k/L is defined as thermal conductance, measured in WK1m2
The ratio L/k is defined thermal resistance R of the material, measured in W-1Km2
Total transmittance U, measured in WK-1 m-2 is the sum of the inverse of the thermal resistance R with the surface resistances i and e (see paragraph 1.2.2 )
If a wall is composed of different layers of materials with different thermal conductivities k1, k2, ... , kn having respective thicknesses s1, s2, sn, its total transmittance is: