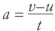If you enjoyed Science Basic Facts, check out these other great Collins Gem titles.

Buy the ebook here

Buy the ebook here

Buy the ebook here
HarperCollinsPublishers
Westerhill Road, Glasgow G64 2QT
www.harpercollins.co.uk
First published by HarperCollinsPublishers 1989
Second edition 1992
Third edition 2002
HarperCollinsPublishers 1989, 1992, 2002
A catalogue record for this book is available from the British Library
Collins Gem is a registered trademark of HarperCollinsPublishers Ltd
All rights reserved under International and Pan-American Copyright Conventions. By payment of the required fees, you have been granted the nonexclusive, nontransferable right to access and read the text of this e-book on-screen. No part of this text may be reproduced, transmitted, downloaded, decompiled, reverse-engineered, or stored in or introduced into any information storage and retrieval system, in any form or by any means, whether electronic or mechanical, now known or hereinafter invented, without the express written permission of HarperCollins e-books.
HarperCollinsPublishers has made every reasonable effort to ensure that any picture content and written content in this ebook has been included or removed in accordance with the contractual and technological constraints in operation at the time of publication.
Source ISBN: 9780007144976
Ebook Edition NOVEMBER 2013 ISBN: 9780007554911
Version: 2013-10-16
Contents
Collins Gem Science is one of a series of illustrated dictionaries of the key terms and concepts used in the most important school subjects. For this edition the text has been updated and colour is now used throughout the book. With its alphabetical arrangement, the book is designed for quick reference to explain the meaning of words used in the subject and so provides a companion both to course work and during revision.
Bold words in an entry identify key terms which are explained in greater detail in entries of their own; important terms that do not have separate entries are shown in italic and are explained in the entry in which they occur. At the back of the book you will find useful Appendices.
The other titles in the series are:
Gem Computers
Gem Chemistry
Gem Physics
Gem Mathematics
Gem Biology
Gem Modern History
Gem Geography
Gem Business Studies
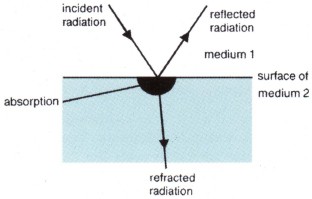
absorption . The taking in of radiated energy. When radiation travelling in one material meets the surface of a second, three things may happen to it.
(a) It may bounce back into the first material: this is called reflection.
(b) It may travel on in a new direction through the second material: this is called refraction.
(c) It may disappear into the second material: this is called absorption.
When a surface absorbs radiation the energy must appear in a new form. In most cases this is heat energy: the temperature increases.
absorption
. (of food) Thatomic nume process by which digested food particles pass from the alimentary canal into the bloodstream. In mammals absorption takes place in the ileum, which is part of the small intestine.
acceleration (a) The change of velocity,v, of an object in unit time. The SI unit of acceleration is the metre per second per second, m/s2. Acceleration can be calculated using the following equation:
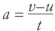
where u is the initial velocity and v is the final velocity. Acceleration is a vector quantity. An object accelerates if its speed and/or its direction of motion change.
When a net outside force, F, acts on an object of mass, m, the resulting acceleration, a, is given by:
a = F/m
At any moment on a velocity/time graph the acceleration is given by the slope of the graph. Positive acceleration results in an increase in velocity, while negative acceleration (often called deceleration or retardation) results in a decrease in velocity.
acid A substance which releases hydrogen ions (H+) when added to water. Acid solutions have a pH of less than 7. Common laboratory acids are:
(a) Strong acids: nitric acid (HNO3), hydrochloric acid (HC1), sulphuric acid (H2SO4) ;
(b) Weak acids: ethanoic acid (CH3COOH), citric acid (C6H8O7).
Strong acids ionize completely in water, weak acids only partially. A few acids are corrosive liquids and must be handled with care.
Acids:
(a) Turn blue litmus red.
(b) Give carbon dioxide when added to carbonates.
(c) Give hydrogen when added to certain metals.
(d) Neutralize alkalis.
acid rain Rain polluted by acids in the atmosphere. Coal often contains substances which produce the acidic gases sulphur dioxide and nitrogen oxides when it is burnt. If these acidic gases are allowed to escape into the atmosphere they dissolve in any moisture present to produce acids. When the moisture falls to the earth as rain it is, in fact, acid(ic) rain.
Acid rain is responsible for considerable damage to the environment. Once soils become highly acidic, plants and animals are adversely affected and potentially harmful ions such as aluminium are able to dissolve in the acidic solution. The acidic solutions may accumulate in lakes where their effects on wildlife are devastating. Recently steps have been taken to reduce the quantity of acidic gases produced by British coalfired power stations and car exhausts. In future it is planned to fit coal-fired power stations with flue desulphurization units to remove acidic gases before flue gases are released into the atmosphere.
active transport The movement of substances against a concentration gradient (see osmosis) using energy produced by metabolic processes. Examples of active transport are (a) the reabsorption of useful substances, including glucose and amino acids, in the mammalian kidney, and (b) the uptake of mineral salts by the root of plant.
air The mixture of gases which make up the atmosphere surrounding the Earth. The exact composition of the air varies slightly from place to place and with height above the ground.
Air, particularly in industrial areas, often contains pollutants. Some common examples are shown below.
| Pollutant | Sources |
| Sulphur dioxide | burning coal and oil |
| Carbon monoxide | engines, cigarettes |
| Nitrogen oxides | burning coal, car engines |