Published in 2010 by The Rosen Publishing Group, Inc.
29 East 21st Street, New York, NY 10010
Copyright 2010 by The Rosen Publishing Group, Inc.
First Edition
All rights reserved. No part of this book may be reproduced in any form without permission in writing from the publisher, except by a reviewer.
Library of Congress Cataloging-in-Publication Data
Hall, Linley Erin.
The transactinides: rutherfordium, dubnium, seaborgium, bohrium, hassium, meitnerium, darmstadtium, roentgenium / Linley Erin Hall.1st ed.
p. cm.(Understanding the elements of the periodic table) Includes bibliographical references and index.
ISBN 978-1-4358-3559-7 (library binding)
1. Superheavy elementsPopular works. I. Title.
QD172.S93H35 2010
546'.449dc22
2009014943
Manufactured in the United States of America
On the cover: The cover graphic shows the eight known transactinide elements, as they appear on the periodic table. The atomic structure of each element is shown.
Contents
Introduction
I n 1869, Dmitry Mendeleyev proposed what he called a periodic table. This chart organized the elements according to their properties. Mendeleyev usually placed elements on the table in order of increasing atomic weight. The elements were also grouped by properties they had in common. But this periodic table contained gaps. Mendeleyev predicted that scientists would discover elements with particular properties to fill those gaps. Over the next two decades, researchers found gallium, scandium, and germanium, which exactly fit gaps in Mendeleyevs table.
Over the years, the periodic table has changed as scientists have learned more about the elements. The table is now organized not by atomic weight but by atomic number. Atomic number is the number of protons (positively charged particles) in an atom of an element. The periodic table also contains many more elements than were known in Mendeleyevs day.
Eventually, scientists discovered all the elements that could be found in nature. So some researchers decided to try to make new elements with larger atomic numbers. They wondered how big an atom could be. They wanted to know if heavier elements would have properties similar to lighter elements on the periodic table. New elements might also have characteristics that would make them useful to humans.
Dmitry Mendeleyev created the first periodic table 140 years ago. The transactinides are the newest additions to the table.
The researchers were successful in making many new elements, although not as successful as many hoped. Still, their work has extended the periodic table. The most recently created elements are the transactinides. The transactinides have atomic numbers of 104 and higher. As far as scientists know, none of the transactinides occur in nature. Researchers have made them all in the laboratory, sometimes with great difficulty. Experiments are occurring even now to create transactinides with higher atomic numbers.
Chemist Paul Karol has calculated that if the historic rate of discovery of new elements (roughly one every two-and-a-half years) continues, the periodic table will include 150 elements by the end of the twenty-first century. Many scientists believe, however, that really heavy elements cannot exist. Only time will tell what the maximum size of the periodic table will be.
Because the transactinides are so new and unstable, little is known about them compared to other elements. But discoveries are made all the time. Research on the transactinides has pushed scientists to create more advanced laboratory equipment that has been used in other experiments as well. This research has also provided new information about atomic structure and properties.
Chapter One
The Transactinides
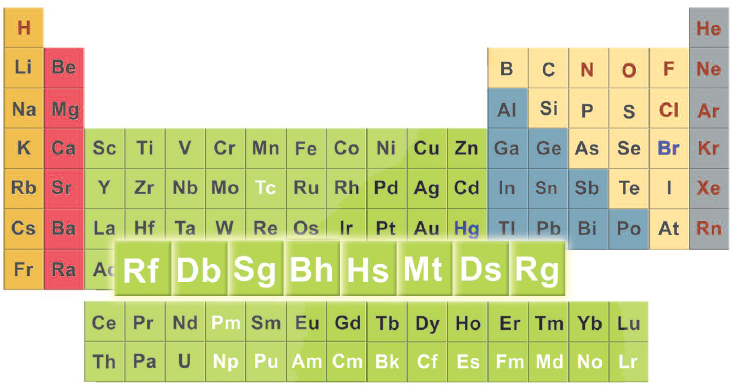
T he transactinides are the elements with atomic numbers of 104 and higher. Their atoms are the largest and heaviest of all the elements. Because of this, the transactinides are sometimes called the superheavy elements. The transactinides that have been named are:
Rutherfordium (Rf): Atomic number 104
Dubnium (Db): Atomic number 105
Seaborgium (Sg): Atomic number 106
Bohrium (Bh): Atomic number 107
Hassium (Hs): Atomic number 108
Meitnerium (Mt): Atomic number 109
Darmstadtium (Ds): Atomic number 110
Roentgenium (Rg): Atomic number 111
Transactinides on the Periodic Table
Most periodic tables have a main block, with two additional rows underneath for the lanthanide and actinide elements. The transactinides that have been discovered so far are found in the last row of the main block of the periodic table. The rows of the table are known as periods. These are numbered from top to bottom.
The modern periodic table contains 111 elements arranged according to their atomic number and properties. The transactinides, or superheavy elements, are found in period (row) 7 and are part of the transition elements.
The transactinides are part of period 7. Within a period, atomic number increases from left to right. There is a jump in atomic numbers in period 7 from 88 to 103 because the actinides listed separately below the table are part of period 7. But the trans in transactinides indicates that these elements have higher atomic numbers than the actinides. Many periodic tables list an elements atomic number and atomic weight (expressed as atomic mass units, or amu). The atomic weights for the transactinides are usually given as whole numbers in parentheses. This indicates that the atomic weights are estimates.
The periodic table can be divided vertically as well as horizontally. The columns of the main block of the table are known as groups. These are numbered from left to right. For example, rutherfordium is a member of group 4, dubnium of group 5, and so on. Elements in a group usually have some similar properties. Because of this, elements in the same column or group are often referred to as homologues. Rutherfordiums homologues in group 4 are titanium, zirconium, and hafnium.
The middle of the main block of the periodic table, groups 3 to 12, are known as the transition elements. All the named transactinides are transition elements. All transition elements are metals. Most are solid at room temperature (mercury is an exception). Unfortunately, because the transactinides have been created in the laboratory only relatively recently (within the past fifty years), researchers dont yet know much about their properties. Chapters 4 and 5 discuss this in more depth.
Some Transactinide History
All the transactinides have been made by scientists in laboratories. Uranium, element 92, is the heaviest element found in nature. All elements, from atomic number 93 on, have been artificially made. Scientists have created most of them in laboratories. Others were first found in the waste from nuclear explosions.
As scientists have learned more about atoms and atomic structure, ideas about the transactinides have changed. In the late 1930s, Lise Meitner and Otto Frisch predicted that elements with atomic numbers larger than 100 couldnt exist. Using what was known about atoms at the time, they calculated that superheavy elements would fall apart immediately. In the 1950s and 1960s, researchers with new information predicted that elements up to atomic number 164 could exist. They thought that some of these superheavy elements would not be stable at all, but that others would be extremely stable. Scientific cartoons from this period show scientists venturing across a sea of instability toward superheavy island.