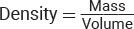Contents
Guide
Page List


Dedication
To
my wife, Patty; children, Emily, Sam, and Ben; granddaughters, Stella and Clare;
grandson, Nate; son-in-law, Rob; and daughter-in-law, Aly
M. Kernion
To
my wife, Jean; daughters, Lisa, Linda, and Lori;
and their families,
who supported my efforts throughout the years
J. Mascetta
Copyright 2022 by Kaplan North America, LLC, dba Barrons Educational Series
Content in this book was previously published in a different format under the title SAT Subject Test in Chemistry.
All rights reserved under International and Pan-American Copyright Conventions. By payment of the required fees, you have been granted the non-exclusive, non-transferable right to access and read the text of this eBook on screen. No part of this text may be reproduced, transmitted, downloaded, decompiled, reverse engineered, or stored in or introduced into any information storage and retrieval system, in any form or by any means, whether electronic or mechanical, now known or hereinafter invented, without the express written permission of the publisher.
Published by Kaplan North America, LLC, dba Barrons Educational Series
1515 W Cypress Creek Road
Fort Lauderdale, FL 33309
www.barronseduc.com
ISBN: 978-1-5062-8151-3
10 9 8 7 6 5 4 3 2 1
Kaplan North America, LLC, dba Barrons Educational Series print books are available at special quantity discounts to use for sales promotions, employee premiums, or educational purposes. For more information or to purchase books, please call the Simon & Schuster special sales department at 866-506-1949.
About the Authors
Mark Kernion, M.A.
Mark Kernion, M.A., taught AP, honors, and academic chemistry for 33 years at Mt. Lebanon High School in Pittsburgh, Pennsylvania, and currently teaches an online AP Chemistry course through PA Homeschoolers and Chemistry-Prep.com. He received the Yale Teaching Award in 2007, was inducted into the Cum Laude Society (dedicated to honoring scholastic achievement in secondary schools) in 2006, and holds a number of patents related to his research.
Joe Mascetta, M.S., C.A.S.
Joe Mascetta, M.S., C.A.S., taught high school chemistry for 20 years. He also served as the science department coordinator and principal of Mt. Lebanon High School in Pittsburgh, Pennsylvania, a science consultant to the area schools, and was a past president of the Western Pennsylvania Association of Supervision and Curriculum Development (ASCD) and the State Advisory Committee of ASCD.
CONTENTS
Barrons Chemistry Practice Plus is designed to offer essential review of key topics and loads of online practice to help you excel in chemistry.
Online Practice
Access more than 400 questions in online quizzes arranged by topic for customized practice! All questions include answer explanations.
What Will You Learn in the Book?
Key review and topics are covered so you can study the essentials needed to succeed.
Learning objectives are listed at the start of each chapter. This list of key ideas will help guide your learning and study plan and allow you to easily return to topics that you want to review again.
Examples are included to help support your learning of surrounding topics.
Tips are given throughout the book to offer helpful notes, reminders, and strategies to improve your learning.
Learning Objectives
In this chapter, you will learn how to:
 Distinguish types of matter: i.e., elements, mixtures, compounds, and pure substances.
Distinguish types of matter: i.e., elements, mixtures, compounds, and pure substances.
 Identify chemical and physical properties and changes.
Identify chemical and physical properties and changes.
 Explain how energy is involved in these changes.
Explain how energy is involved in these changes.
 Identify and use the SI units of measurements.
Identify and use the SI units of measurements.
 Do mathematical calculations by using scientific notation, dimensional analysis, and proper significant figures.
Do mathematical calculations by using scientific notation, dimensional analysis, and proper significant figures.
Chemistry is the study of matter and how it changes. Changes in matter are always accompanied by changes in the energy associated with the matter that is changing. Therefore, chemistry investigates matter and energy as well as the changes they undergo as processes unfold. A good understanding of both matter and energy is thus required to understand the world from a chemical perspective.
Matter is defined as anything that occupies space and has mass. Mass is the quantity of matter that a substance possesses and, depending on the gravitational force acting on it, has a unit of weight assigned to it. Its formula is w = mg, where m is the mass of the substance and g is a gravitational constant. Although weight can vary as the gravitational constant does, the mass of the body is a constant and can be measured by its resistance to a change of position or motion. This property of mass to resist a change of position or motion is called inertia. Since matter does occupy space, we can compare the masses of various substances that occupy a particular unit volume. This relationship of mass to a unit volume is called the density of the substance. It can be shown in a mathematical formula as 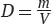 . The unit of mass (m) commonly used in chemistry is the gram (g), and of volume (V) is the cubic centimeter (cm3), milliliter (mL), or liter (L).
. The unit of mass (m) commonly used in chemistry is the gram (g), and of volume (V) is the cubic centimeter (cm3), milliliter (mL), or liter (L).
TIP
Matter occupies space and has mass.
An example of how density varies can be shown by the difference in the volumes occupied by 1 gram of a metal, such as gold, and 1 gram of Styrofoam. Both have the same mass, 1 gram, but the volume occupied by the Styrofoam is much larger. Therefore, the density of the gold is much larger than that of the Styrofoam. In chemistry, the typical unit for the density of solids is grams/cubic centimeter and for that of liquids is grams/milliliter. Both of these are temperature dependent. The typical unit for the density of gases is grams/liter at standard temperature and pressure. However, the density of gases is usually much lower than the densities of solids and liquids, which is the reason for the unit of liter in the denominator. This ensures a reasonable amount of mass will be reported for gases. Density is an important differentiating property of matter. It is a quantitative value that can be assigned to all matter based on the definition of matter: anything that has mass and volume.
TIP
States of Matter
Matter occurs in three states: solid, liquid, and gas. A

















 Distinguish types of matter: i.e., elements, mixtures, compounds, and pure substances.
Distinguish types of matter: i.e., elements, mixtures, compounds, and pure substances.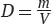 . The unit of mass (m) commonly used in chemistry is the gram (g), and of volume (V) is the cubic centimeter (cm3), milliliter (mL), or liter (L).
. The unit of mass (m) commonly used in chemistry is the gram (g), and of volume (V) is the cubic centimeter (cm3), milliliter (mL), or liter (L).