Contents
Guide
Page List


Dedication
I wish to dedicate this book to
My wonderful children, Michael and Sara Boilen, who I love and cherish
Acknowledgments
I wish to thank
My husband, Howard Blue, for his constant love and support
I wish to thank
My husband, Sheldon, for his constant and unwavering love, encouragement, and support
My parents for their support of my pursuit of a career in science and education
Copyright 2022 by Kaplan North America, LLC, dba Barrons Educational Series
Content in this book was previously published in a different format under the title SAT Subject Test in Biology.
All rights reserved under International and Pan-American Copyright Conventions. By payment of the required fees, you have been granted the non-exclusive, non-transferable right to access and read the text of this eBook on screen. No part of this text may be reproduced, transmitted, downloaded, decompiled, reverse engineered, or stored in or introduced into any information storage and retrieval system, in any form or by any means, whether electronic or mechanical, now known or hereinafter invented, without the express written permission of the publisher.
Published by Kaplan North America, LLC, dba Barrons Educational Series
1515 W Cypress Creek Road
Fort Lauderdale, FL 33309
www.barronseduc.com
ISBN: 978-1-5062-8149-0
10 9 8 7 6 5 4 3 2 1
About the Authors
Deborah Goldberg, M.S.
Deborah Goldberg, M.S. taught AP Biology and Chemistry at Lawrence High School on Long Island, New York for more than 20 years. She also spent 14 years completing research as an electron microscopist at New York University Medical Center and New York Medical College.
Marisa Abrams, M.S.
Marisa Abrams, M.S. has taught AP Biology, Biology, and Chemistry for 19 years. She has attended the AP Biology Read (as both a reader and a Table Leader), and she has worked with ETS and the College Board in various capacities (item writing/reviewing for AP and serving on Biology committees).
CONTENTS
Barrons Biology Practice Plus is designed to offer essential review of key topics and loads of online practice to help you excel in biology.
Online Practice
Access more than 400 questions in online quizzes arranged by topic for customized practice! All questions include answer explanations.
To access these questions, see the card at the front of the book.
What Will You Learn in the Book?
Key review and topics are covered so you can study the essentials needed to succeed.
Learning objectives are listed at the start of each chapter. This list of key ideas will help guide your learning and study plan and allow you to easily return to topics that you want to review again.
Tips are given throughout the book to offer helpful notes, reminders, and strategies to improve your learning.

Learning Objectives
In this chapter, you will learn:
To understand about living things, you must understand some basic biochemistry. Biochemistry affects every aspect of our lives every day. Sweating cools the skin because of strong hydrogen bonding between water molecules. Your body maintains your blood at one critical pH because of the bicarbonate buffering system. To prevent heart attacks, you must begin with an understanding about the structure of fatty acids. Mad cow disease is caused by a misfolded protein. Here is a review of basic biochemistry.
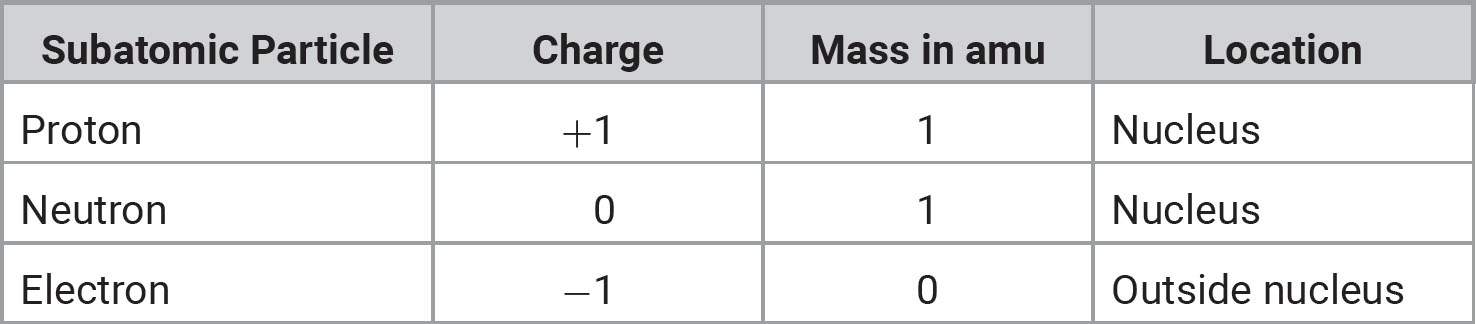
The atom consists of subatomic particles: protons, neutrons, and electrons. compares these particles.
TABLE 1.1 Subatomic Particles
1.An atom in the elemental state always has a neutral charge because the number of protons (+) equals the number of electrons ().
2.Electron configuration is important because it determines how a particular atom will react with atoms of other elements.
3.Electrons in the lowest available energy level are said to be in the ground state.
4.When an atom absorbs energy, its electrons move to a higher energy level. The atom is then said to be in the excited state. For example, during photosynthesis, chlorophyll molecules absorb light energy, which boosts electrons to higher energy levels. These excited electrons provide the energy to make sugar as they return to their ground state and release the energy they previously absorbed.
CAREFUL
Dont confuse isotope with isomer.
Isotopes are atoms of one element that vary only in the number of neutrons in the nucleus. Chemically, all isotopes of the same element are identical because they have the same number of electrons. For example, carbon-12 and carbon-14 are isotopes of each other and are chemically identical. They both possess 6 protons and 6 electrons. However, carbon-12 has 6 neutrons, while carbon-14 has 8 neutrons. Some isotopes, like carbon-14, are radioactive (called radioisotopes). The nuclei of radioisotopes emit particles and decay at a known rate called a half-life. Knowing the half-life enables us to measure the age of fossils or to estimate the age of Earth.
Radioisotopes are useful in other ways. Besides measuring the age of fossils, they can be used in medical diagnosis, treatment, and research. For example: radioactive iodine (I-131) can be used both to diagnose and to treat certain diseases of the thyroid gland. In addition, radioactive carbon can be used as a tracer, incorporated into molecules of carbon dioxide, and used to track metabolic pathways.
A bond is formed when two atomic nuclei attract the same electron(s). Energy is released when a bond is formed. Energy must be supplied or absorbed to break a bond. Atoms bond to achieve stability, to acquire a completed outer shell. There are two main types of bonds, ionic and covalent.
Ionic bonds form when electrons are transferred. An atom that gains electrons becomes an anion, which stands for a n egative ion . An atom that loses an electron becomes a cation, a positive ion. Ions such as Cl, Na+, and Ca2+ are necessary for normal cell, tissue, and organ function.
Covalent bonds form when atoms share electrons. The resulting structure is called a molecule. A single covalent bond () results when two atoms share one pair of electrons. A double covalent bond (=) results when two atoms share two pairs of electrons, and a triple covalent bond () results when two atoms share three pairs of electrons.
There are two types of covalent bonds, nonpolar and polar. This classification is based on whether electrons are shared equally or unequally. See .
TABLE 1.2


















