1.1 Introduction
The dermal-epidermal basement membrane zone (BMZ) is a complex, precisely organized, and dynamic collection of proteins which provide structure and regulate cell adhesion, differentiation, motility, signal transmission, and membrane permeability. The complex roles of the proteins of the BMZ are especially important in development, wound healing, and neoplastic progression. Understanding the structure and function of each individual protein will provide insight on how the supramolecular complexes of proteins are involved in human tissues and diseases, in particular inherited blistering diseases known as epidermolysis bullosa and acquired autoimmune processes such as the pemphigus and pemphigoid families of blistering diseases.
Traditionally, PAS (periodic acidSchiff) stain is used to histologically enhance the region of the dermal-epidermal junction. However, the PAS stain enhances regions far beyond the normal boundaries of the BMZ, leading to a widespread and totally incorrect impression that the BMZ is visible by light microscopy. In fact, the dermal-epidermal BMZ, which consists of an electron-dense lamina densa and an electron-lucent lamina lucida, is far too small to be visualized by light microscopy and can only be truly visualized by electron microscopy. Together, the 50 mm lamina densa and the 40 nm lamina lucida form the basal lamina []. These two layers form the BMZ of most tissues; however, tissues such as the skin, portions of the GI, GU, and respiratory mucosa, cornea, and amnion have developed additional specialized BMZ elements to augment dermal-epidermal cohesion in the face of disruptive external forces.
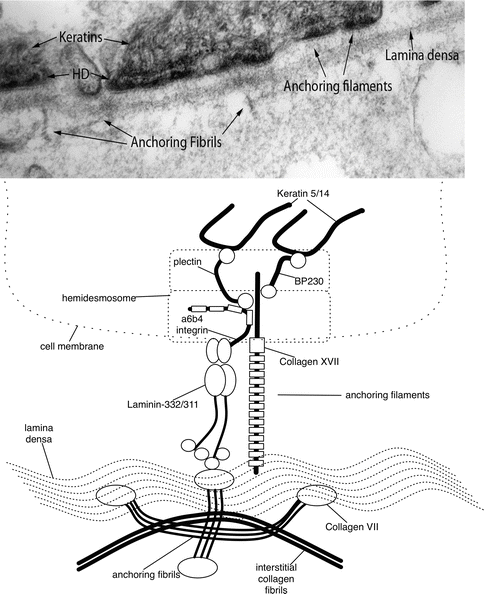
As can be seen in Fig. ].
Fig. 1.1
Biochemical composition of dermal-epidermal basement membrane ultrastructural entities. Ultrastructural entities viewed by transmission electron microscopy ( above ) compared with a schematic ( below ) revealing their biochemical composition
Newer techniques have been developed that allow for visualization of the BMZ with greater physiological accuracy []. High-pressure freeze substitution allows for high-quality tissue preservation. This has illustrated that the conventional dermal-epidermal interface may in fact be inaccurate and likely an artifact secondary to tissue preparation. When tissue is dehydrated in preparation for electron microscopy fixation, the plasma membrane may be separated, exposing the lamina lucida. Therefore, the lamina lucida may be absent physiologically and the lamina densa may actually hold the anchoring filaments. From this insight, arguments about whether anchoring filament proteins reside in the lamina lucida or lamina densa become superfluous. Since conventional electron microscopy is still widely used to visualize the BMZ, conventional terms will still be used in this chapter.
The BMZ consists of complex and dynamic interactions between both intracellular and extracellular proteins, as shown in Fig. ].
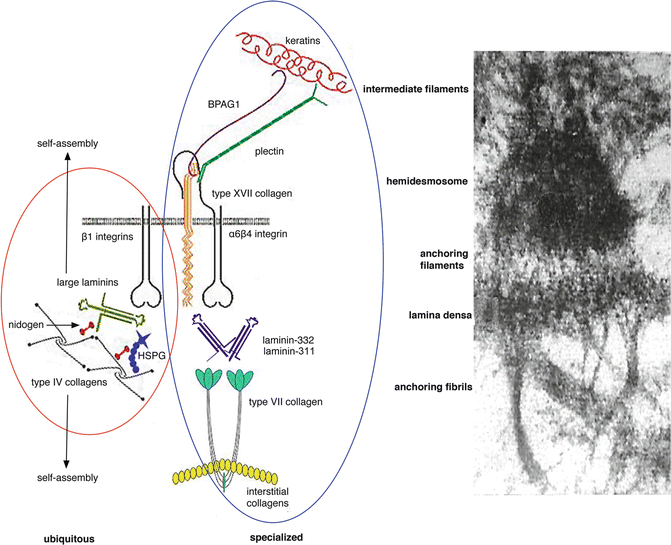
Fig. 1.2
Ubiquitous and specialized components of the dermal-epidermal basement membrane. All basement membranes (including the dermal-epidermal basement membrane) contain a group of ubiquitous components ( red circle ). These include large laminins such as laminin-511, type IV collagen, the heparan sulfate proteoglycan perlecan (HSPG), and nidogen. These molecules undergo a self-assembly process to form the basal lamina. In addition, stratified squamous, corneal, amniotic, and certain other epithelial tissues exposed to disruptive external forces contain a number of specialized components ( blue circle ) including keratins 5 and 14, keratin linker proteins BPAG1 and plectin, transmembrane proteins type XVII collagen, [alpha]6[beta]4 and [alpha]3[beta]1 integrins, specialized laminins such as laminin-332 and laminin-311, and type VII collagen. Electron microscopic image is included to the right for comparison
Newer techniques continue to illustrate the role of these ubiquitous proteins. For example, using immunogold electron microscopy and immunoblotting, perlecans were found to be key organizing proteins, connecting type IV collagen and laminins [].
Specialized proteins are also found in tissues that are subject to external disruptive forces and need additional cohesion. Perhaps the most studied example of this type of specialized BMZ is the dermal-epidermal basement membrane zone. Starting with the most superior aspects, plectins, which are keratin linker proteins, and BPAG1, also known as BP230, are specialized intracellular proteins of the dermal-epidermal BMZ. These proteins are connected to transmembrane proteins and together form the hemidesmosome. Transmembrane proteins of the hemidesmosome include integrin [alpha]6[beta]4 and type XVII collagen, also known as BP180 or BPAG2. Both of these proteins also connect to extracellular proteins laminin-332 and laminin-311, together forming the anchoring filaments. The extracellular proteins also connect to the ubiquitous proteins that were discussed above to form the lamina densa. Extending perpendicular from the lamina densa are thick-banded structures, known as the anchoring fibrils. The anchoring fibrils, composed of type VII collagen, loop through the dermal interstitial collagen fibrils and reattach back onto the lamina densa. New three-dimensional human skin models are now being utilized to further investigate the BMZ [].
The basement membrane zone is an intricate structure of ubiquitous and specialized proteins that together are key to preserving tissue structure and function. Understanding each element will illustrate the complexity and importance of the BMZ as a whole.
1.2 Ubiquitous BMZ Components
1.2.1 Nidogen/Entactin
Nidogen, also known as entactin, is a ubiquitous glycoprotein which is both a member of the BMZ and important in linking a variety of other proteins, including laminin and type IV collagen [].
The absence of one of the two nidogens does not affect BMZ formation; however, the BMZ can be completely lost if both are absent, such as in the capillary or skin BMZ [].
1.2.2 Type IV Collagen
Type IV collagen, which may comprise more than half of the mass of BMZ, is a heterotrimer containing distinct alpha chains. There are six genes that encode six different 400 nm isoforms ([alpha]1-[alpha]6). The isoforms have an N-terminal 7S domain (26 kDa, 28 nm), a triple helical collagenous domain (120 kDa, 320 nm) made from repeats of glycine-proline/hydroxyproline-lysine/hydroxylysine, and a C-terminal noncollagenous globular domain known as NC1 (25 kDa, 52 nm). The NC1 and 7S domains are key to the formation of the collagen network, while the triple helical domain is important in the integrity and flexibility of type IV collagen [].