1.1.1 Bone Function and Structure
Bone is the major constituent of the skeleton which is a hallmark of all higher vertebrates. Besides the protection of internal organs and the support of body structures, the most important functions of bone are to serve as an attachment site for ligaments, tendons, and muscles allowing locomotion and provide a cavity for hematopoiesis in the bone marrow (Mendez-Ferrer et al. ). Moreover, bone has a central role in mineral homeostasis as it functions as a reservoir for inorganic ions that can be mobilized rapidly on metabolic demand.
Although bone is often considered an inert, static material, it is a highly organized, living tissue that undergoes constant remodeling. Different cell lineages have emerged to serve distinct skeletal functions. While cells from the hematopoietic lineage, such as osteoclasts, break down bone tissue to remove old and damaged bone or release calcium to maintain calcium homeostasis, cells from the mesenchymal lineage, including chondroblasts, fibroblasts, and osteoblasts, construct and later remodel bone tissue (Jiang et al. ). Osteoblasts produce the organic components of the extracellular matrix, which mainly includes type I collagen (approximately 95%), but also noncollagenous proteins (i.e., osteocalcin, osteopontin, osteonectin, bone sialoprotein) and proteoglycans. The inorganic matrix predominantly contains calcium and phosphorus, appearing as hydroxyapatite crystals (Ca10(PO4)6(OH)2), and is deposited into the collagenous matrix. This complex organization confers rigidity and strength to the skeleton while maintaining a high degree of elasticity.
Two types of osseous tissues are found in all bones: cortical or compact bone and trabecular or cancellous bone, sometimes also referred to as spongy bone (Fig. ). Cortical bone is mainly found in the shafts of long bones (diaphyses) and is made of numerous overlapping cylindrical units termed Haversian systems or osteons. The central Haversian canal, containing the blood vessel and nerves, is surrounded by densely packed collagen fibrils which are formed to concentric lamellae. Osteocytes, terminally differentiated osteoblasts, are located between concentric lamellae and are connected to each other via canaliculi, allowing the exchange of nutrients and metabolic waste and the sensation of mechanical stress. Volkmanns canals are responsible for the conjunction of blood vessels from the inner and outer bone surfaces to the vessels of the Haversian canals. The dense organization of cortical bone thus provides maximum strength and load-bearing capacity by being highly resistance to bending and torsion. Cancellous bone, on the other side, is predominantly found at the ends of long bones (epiphyses) as well as in flat bones and in vertebral bodies where force may be applied at variable angles. It is composed of a meshwork of trabeculae, thereby reducing skeletal weight without compromising strength. This particular construction also establishes a vast surface area. Considering that bone remodeling only takes place at bone surfaces, cancellous bone is quick to render metabolic activities but also disproportionally susceptible to damage when net bone loss occurs.
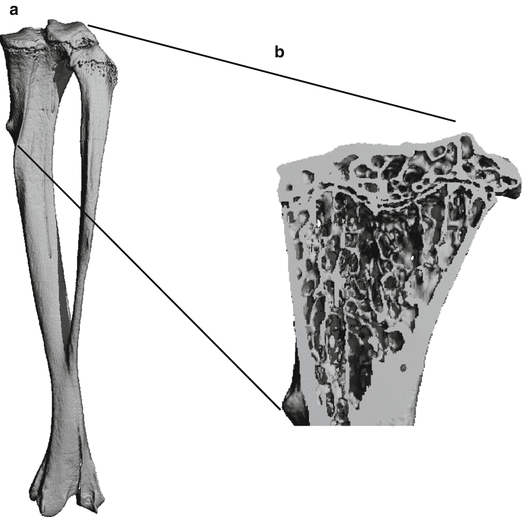
Fig. 1.1
Illustration of compact and cancellous bone. ( a ) Whole tibial structure measured by micro-computed tomography. Twelve-micron isotropic spatial resolution. ( b ) Lateral view of the tibia. The trabecular meshwork structure is clearly visible at the epiphysis. Cortical bone is found at the shaft ( Source : Peter Varga and Phillipe Zysset)
1.1.2 Ossification Processes
Ossification occurs either intramembranous or endochondral. During skeletal development, flat bones (e.g., calvariae) and some irregular bones are formed by intramembranous ossification where bony tissue directly forms from the connective tissue without an intermediate cartilage stage (Blair et al. ).
In contrast to flat and irregular bones, bones of the vertebral column, pelvis, and extremities develop by endochondral ossification. Thereby, hyaline cartilage devoid of blood vessels is first formed and then replaced by bone matrix starting at the primary ossification center. During embryonic development, chondrocytes congregate to a cartilaginous model that alleges the shape of the future bone, and after the local enlargement of chondrocytes (hypertrophy), endochondral bone formation is initiated in the middle of the shaft at the primary ossification center. The perichondrium, which surrounds the cartilage model, becomes invaded with blood vessels and then is called periosteum (Stanka et al. ).
The growth plates are characterized by the orderly proliferation and maturation of chondrocytes in longitudinal columns, forming stratified zones of reserve, proliferative, maturing, and hypertrophic cartilage (Poole et al. ). By doing so, transverse septa of cartilage matrix surrounding them are broken down, leaving vertical septa largely intact, but allowing the entry of capillaries and invading cells of the ossification front. These cells mainly include cells of the mesenchymal (osteoblast precursors and stromal cells) and hematopoietic lineages (osteoclast precursors and other hematopoietic lineages that constitute the bone marrow). After osteoblast precursor cells have migrated to the surface of remnant cartilage spicules, they differentiate into fully mature osteoblasts and deposit a predominantly type I collagen-containing extracellular matrix (osteoid), which subsequently becomes mineralized into the mature bone matrix. The ossification continues toward the ends of the bones, where the further elongation of long bones occurs in the growth plates of the metaphysis. Finally, the trabecular bone in the diaphysis is broken down by osteoclasts to open up the medullary cavity.
The same procedure is true for the secondary ossification center, located in the epiphysis, except that the trabecular bone is retained (Alini et al. ). The cartilage in the epiphyseal plate remains proliferating and is continuously replaced by bone matrix until the skeleton has reached maturity and the epiphyseal plate has become almost completely ossified. The articular cartilage remains uncalcified and covers the ends of the long bones. Due to its incredibly low coefficient of friction, coupled with its ability to bear very large compressive loads, articular cartilage is ideally suited for placement in joints, such as the knee and hip.