In order to furnish extremely thin, absolutely even sections, you require a special apparatus, the microtome. With the help of this slicing machine you can make slices without holes of such a thinness as you could never get by hand cuttings, even with much practice. Naturally you can see much more in a series of sections than in single cells; in this way it is possible, for instance, to reconstruct the entire inner structure of an organ by means of a series of sections.
Microtomes are precision instruments and are therefore expensive; nevertheless for many purposes a simple hand microtome is sufficient, as described on page 99. It can however never be too strongly impressed that every microscopist should learn the manual cutting technique thoroughly before he attempts to make microtome sections. The feeling for every cutting operation can best be obtained by becoming practised in the use of the hand razor. Besides, in many cases, particularly with objects from the plant world, hand cuttings are sufficient even for scientific purposes.
If you wish to section any object on the microtome, you must first prepare it in a definite manner. Most of the materialswhether they be of animal or plant originare much too soft to be cut without any further preparation. You must thereforeafter previous painstaking fixingembed them in paraffin or celloidin, if you do not prefer to give them sufficient hardness by freezing. First you must acquaint yourself with the preparation of objects for microtome operation.
FIXING
Plant and animal structures which have been removed from their natural surroundings change so quickly that in a short time they are no longer suitable for microscopic examination. This process, known as autolysis, you must try to slow down. As soon as they are removed from the organism, place the tissues to be examined in a fluid which kills the cells and at the same time preserves their structures as naturally as possible. From the multitude of known fixing solutions, select a few which have proved themselves for decades; almost all will give good results to start with.
When fixingas with all the other subsequent tasksyou must work with great caution. Sloppy work only leads to failures!
In order that the fixing fluid will penetrate the object equally from all sides, place it in a small glass vessel filled with the solution covering a little glass-wool. The volume of the fixing solution should be at least 50 times that of the object to be fixed.
The time required for fixing varies greatly, depending on the nature of the fixed object. You must be careful that the bit of organ is fixed all the way through without being left too long in the fluid. Larger objects are much more difficult to fix and later are more tedious to section than small ones. If possible, it is therefore best to choose pieces of from 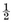 cm. to 1 cm. in thickness.
cm. to 1 cm. in thickness.
The most easy-to-use fixing solution is formalin, the 40% solution of formaldehyde in water. The commercially sold formalin is thinned with tap water at a proportion of 1 :4 and the objects are left in it from one to several days, depending on size. After this they are washed in several changes of 50% alcohol or in water for 2 to 3 days.
Excellent results can be achieved with Bouins fixing mixture. To prepare it, mix together, just before using, 15 cc. of saturated aqueous picric acid solution, 5 cc. formalin and 1 cc. of acetic acid and fix smaller objects in this for 2 hours, larger ones 1 to 2 days. After fixing, the objects are washed for 1 to 2 days in 70% alcohol which is changed 3 times.
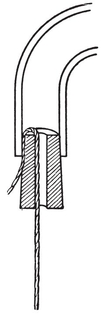
For examining the cytoplasmic structures, a very good substance is potassium bichromate-acetic acid. Shortly before using, mix 100 cc. of potassium bichromate solution with 5 cc. of acetic acid and fix in this for 24 to 48 hours. In this case, wash with tap water, at best in running water for at least 12 hours. If the constantly splashing sound of running water is disturbing, help can be given as shown in : a cork with a hole drilled in it is placed in the tap and a thread runs from the cork to the surface of the water. The water runs along the string with scarcely a sound if the tap is not turned on too far.
After fixing the organic materials in potassium bichromate solution, and until you embed them in paraffin or celloidin, they must be kept in the dark (as in a cabinet) because light causes bothersome precipitations.
There are various sublimate mixtures among the most commonly used fixing materials. Because corrosive sublimate (chloride of mercury) is a dangerous poison, its use requires great caution. Very good results follow the proper use of acetic acid sublimate, a mixture which is indicated especially in embryological examinations. Mix 100 cc. of saturated sublimate solution with 5 cc. of acetic acid to fix very small objects for half an hour, larger ones up to 24 hours.
The subsequent treatment of objects fixed in sublimates is somewhat complicated. Wash in 70% alcohol and transfer to 80% alcohol to which enough iodine in aqueous potassium iodide solution has been added to turn the color to that of strong tea. When fixing in fluids which contain sublimates there are formed so-called sublimate precipitations which not only bother the microscopist but can even lead to failures. By iodizing, these precipitations are eliminated.
DEHYDRATION AND PARAFFIN INFILTRATION
Before you can embed the organic fragments in paraffin or celloidin, they must be completely dehydrated. To do this, substitute the water which remains in the tissues with alcohol. If you put your objects in absolute alcohol at once, however, the concentration balance between water and alcohol would cause distortions, shrinkages and even damages in the tissues. You must therefore dehydrate in stages, whereby you transfer the object from water to 30, 40, 50, 70, 80 and 95% alcohol. The lower stages (up to 70%) should last at least 4 hours, and the higher ones at least 12 hours. Here the size of the object also governs the time of immersion. By using a fixing solution which is washed out with alcohol, dehydration is already begun with the washing process. Then continue with the next higher stage.
In place of the alcohol stages you can dehydrate in one step with methyl glycol. From methyl glycol you can transfer immediately to absolute isopropyl alcohol.
As with the fixing fluid, the alcohol must come in contact with all surfaces of the organic material. Therefore use glass-wool again or, better yet, hang it in the upper layers with a bit of thread. Of course all the containers used should be sealable, or at least be covered with a piece of glass.
From the 95% alcohol transfer your objects to absolute alcohol. However, absolute ethyl alcohol is very hygroscopic (water-absorbent) so that it is difficult to keep it free of water. For this reason you will do better to use absolute isopropyl alcohol, which has other advantages as well over ethyl alcohol.
The absolute isopropyl alcohol is changed three times. Total time of immersion in absolute alcohol should be 2 to 3 days.
Because isopropyl alcohol is poorly soluble in paraffin, do not transfer the organic bits directly from absolute alcohol to paraffin, but inject an intermediary stage between alcohol and paraffin. Many experienced histologists prefer benzol as the best intermediary. The beginner will probably get better results with pure turpentine than with such quickly evaporating substances as benzol and chloroform.













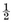 cm. to 1 cm. in thickness.
cm. to 1 cm. in thickness.