For my father, who introduced me to science and
let me peer into a microscope before I could even read
Published in 2010 by The Rosen Publishing Group, Inc.
29 East 21st Street, New York, NY 10010
Copyright 2010 by The Rosen Publishing Group, Inc.
First Edition
All rights reserved. No part of this book may be reproduced in any form without permission in writing from the publisher, except by a reviewer.
Library of Congress Cataloging-in-Publication Data
Furgang, Adam.
The noble gases: helium, neon, argon, krypton, xenon, radon / Adam
Furgang.1st ed.
p. cm.(Understanding the elements of the periodic table)
Includes bibliographical references and index.
ISBN 978-1-4358-3558-0 (library binding)
1. Gases, RarePopular works. 2. AtomsPopular works. 3. Periodic lawPopular works. I. Title.
QD162.F87 2010
546'.75dc22
2009014409
Manufactured in the United States of America
On the cover: The six noble gases, as they appear on the periodic table. The atomic structure of each element is shown.
Contents
Introduction
H ave you ever looked around you and wondered where everything comes from? What is all the matter that you see in your environment made of? Every substance and material around youevery living and nonliving thing that you see every dayis made up of chemical elements.
Chemical elements are the simplest form that matter can be broken down into. When you talk about elements, you are really talking about atoms and how they are arranged. An atom is the smallest form of matter that exists. It cannot be broken down any further by chemical reactions. There are more than ninety elements found naturally on Earth. In addition to these elements, there are more than twenty that are made in laboratories. Every other substance on Earth is made of a combination of these elements.
An element in its pure form will only be made up of atoms from that element. For example, gold (Au) will only have gold atoms in it. Mercury (Hg) will only have mercury atoms in it. Some elements, such as silver (Ag) and iron (Fe), are commonly known and used in everyday life. Others elements, such as francium (Fr) or berkelium (Bk), are only known about, used, and understood by scientists. Some elements are commonly found all over Earths surface, while others are rare and found only in small amounts in nature.
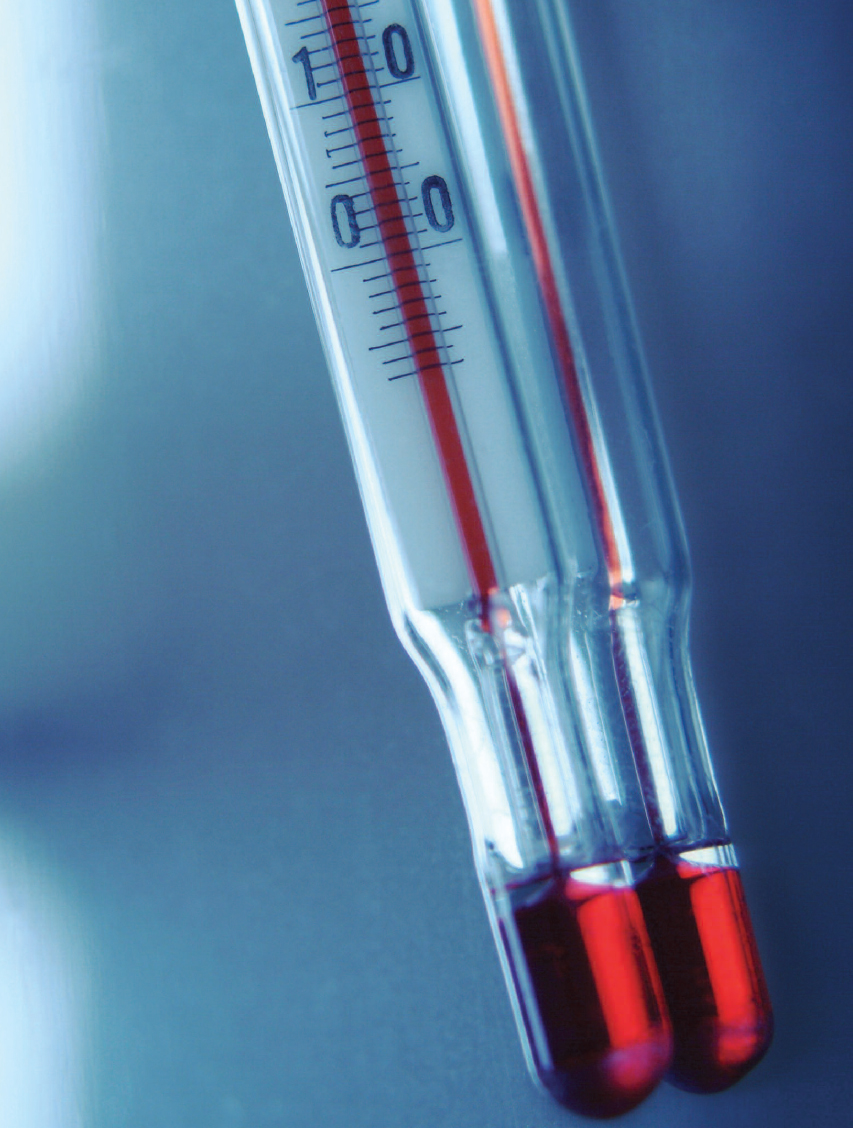
Most thermometers are filled with liquid mercury, an element for which many practical uses have been found.
How do scientists group elements? First, they look at the atoms that make up an element. Each atom has its own structurean arrangement of parts that makes each element unique. The arrangement of the parts of an atom in any single element gives that element its own special properties and characteristics.
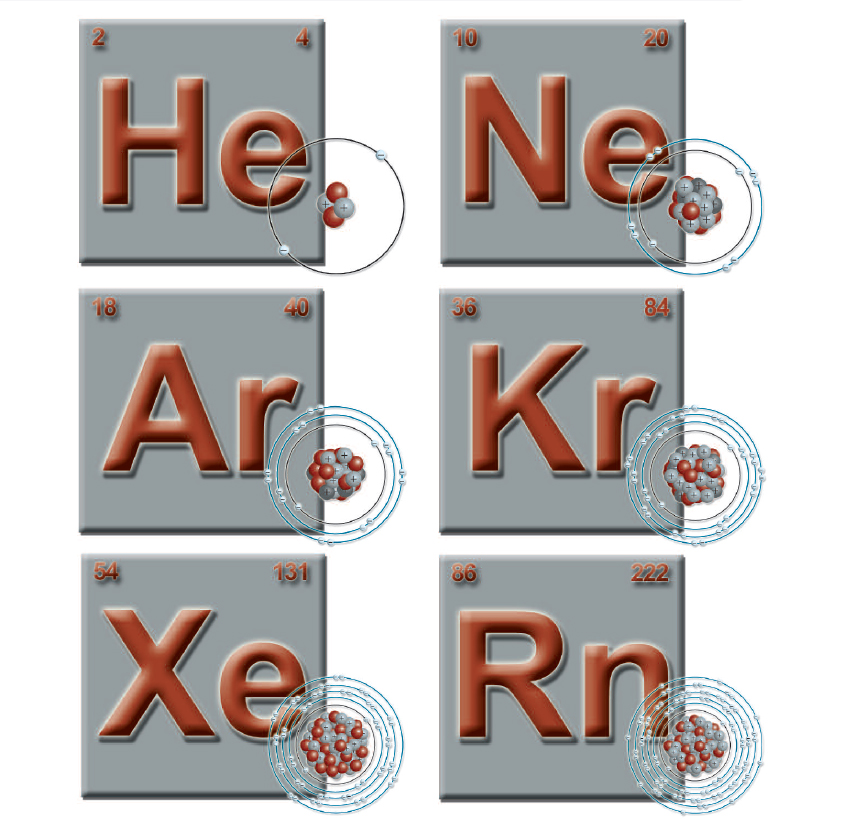
Among all the many elements, there are six elements that are gases and that are grouped together by scientists. These are the noble gases. The noble gases are a unique grouping of elements found naturally on Earth. These elements are helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), and radon (Rn).
The noble gases are mixed in with the air that we breathe. Some of the gases are more common than others. For example, helium is one of the most common elements in the universe, but it is found only in limited amounts in Earths atmosphere. Throughout this book, you will learn about what makes the noble gases unique. You will also learn about how their atoms are arranged and how they are used in industry, our daily lives, and scientific research.
Chapter One
Elements and Their Parts
T ounderstand how one element differs from another, you must first understand what makes up an atom. By performing many experiments, scientists have been able to discover different parts of an atom and uncover some of the mysteries of what makes each element unique.
Protons, Neutrons, and Electrons
Atoms make up elements. They are very small, but they are not the smallest known particles. Atoms themselves are made up of even smaller particles. If you could go to the center, or core, of each atom, you would find its nucleus. The nucleus is made of tiny particles called protons and neutrons. Protons have a positive electrical charge, while neutrons have no electrical charge. Each element has a different number of protons at its core. This number of protons in an atom of an element is called its atomic number.
If you look at the outer shell of an atom, you will find tiny particles called electrons moving around inside the atom. The number of electrons in an atom matches the number of protons in that atoms nucleus, but the electrons have a negative electrical charge.
These are the symbols that represent the six noble gases. Each element shown here has an atomic number, an atomic weight, and a visual representation of its atom.
For example, the atomic number of hydrogen (H) is 1. That means a hydrogen atom has one proton and one electron. The atomic number of gold is 79. That means a gold atom has seventy-nine protons and seventynine electrons.
Each element has a different arrangement of electrons around the nucleus. The arrangement of electrons forms a kind of shell around the atom. The outer shell of an atom, where the electrons are, is about ten thousand times larger than the nucleus. What holds the parts of an atom together? An electromagnetic force holds an atom together and keeps it from flying apart. This is similar to the gravitational force that keeps objects around us firmly on the ground. The same force keeps the planets in a stable orbit, rather than spinning off on a wild trajectory. It is the atoms that give mass to every substance around us. The atoms of different elements have different masses. This is called the elements atomic mass. The atoms of lead (Pb) are much heavier than the atoms of aluminum (Al), and this is why a piece of lead is much heavier than a piece of aluminum of the same size.
Dmitry Mendeleyev
Dmitry Mendeleyev was born in Siberia, Russia, in 1834. He was the youngest in a family that had as many as fourteen children. While Mendeleyev was finishing high school, his father died. The familys glass factory burned down soon after. His mother took him to St. Petersburg, where he enrolled in the Main Pedagogical Institute. He studied science and graduated in 1856. Mendeleyev is best known for his work on the periodic table of elements. In 1869, he presented a paper before the Russian Chemical Society in which he introduced his table of the sixty-three known elements. Each element was listed according to its atomic mass. The basis of his work is still in use today in the modern periodic table. Mendeleyev died in 1907.
Getting Organized
Because there are so many elements, each with its own properties, scientists began trying to organize them in some logical way.
Russian scientist Dmitry Mendeleyev is credited with creating the first periodic table that logically arranged the known chemical elements. First, he arranged similar elements together. Eventually, he organized the elements according to each ones atomic mass. The modern periodic table is still based on Mendeleyevs original work. Today, the periodic table is slightly different from Mendeleyevs because now the elements are listed in order of increasing atomic number. The number of protons that each elements atoms have corresponds to the elements atomic number on the periodic table.