Page List

Published in 2009 by The Rosen Publishing Group, Inc.
29 East 21st Street, New York, NY 10010
www.rosenpublishing.com
Copyright 2009 by The Rosen Publishing Group, Inc.
First Edition
All rights reserved. No part of this book may be reproduced in any form without permission in writing from the publisher, except by a reviewer.
Library of Congress Cataloging-in-Publication Data
Levy, Janey.
Krypton / Janey Levy.
p. cm.(Understanding the elements of the periodic table)
Includes bibliographical references and index.
ISBN-13: 978-1-4042-1778-2 (lib. bdg.)
1. Krypton. 2. Periodic lawTables. I. Title.
QD181.K6L48 2008
546.754dc22
2007040005
Manufactured in the United States of America
On the cover: Kryptons square on the periodic table of elements. Inset: The atomic structure of krypton.
Contents
T he word krypton may make you think of Superman. Krypton was the name of his home planet, and kryptonite was the mineral that could drain his superpowers. Given its association with a fictional superhero, it may surprise you to learn there really is a krypton (Kr). It is not a planet, though. It is a chemical element, a gas that belongs to a group of gases called the noble or inert gases.
The discovery of krypton resulted from efforts to answer a scientific puzzle: why was nitrogen (N) obtained from air heavier than nitrogen obtained from chemical compounds? Any scientist will tell you it is common for research into one topic to give rise to questions that lead to new topics. Seeking answers to those new questions is one of the important ways scientific knowledge grows. The nitrogen puzzle was worth solving because of nitrogens importance. Nitrogen is a colorless, odorless, and tasteless gas that makes up about 78 percent of Earths atmosphere. It is necessary for life as we know itall organisms must have nitrogen to live.
Scottish chemist Sir William Ramsay, assisted by British chemist Morris Travers, began a series of experiments in an effort to solve the puzzle about nitrogen. They succeeded, and in the process, discovered krypton.
Their discovery advanced scientific knowledge and gave us more information about the atmosphere that surrounds us. However, it didnt immediately lead to any practical applications for krypton. Scientists believed that krypton and the other noble gases were inert, or nonreactive. In an article in The Chemical Educator, Professor George Kauffman wrote that chemists used to have a joke about the noble gases: if someone wrote a book titled The Chemistry of the Inert Gases, it would be filled with blank pages. It was not until more than sixty years after kryptons discovery that scientists learned they were wrong and the noble gases could do interesting things.
Theodore Gray and Max Whitby build museum displays of chemical elements. They created this version of kryptons square on the periodic table. Handmade discharge tubes form kryptons chemical symbol.
In the last half century, scientists have discovered many possible uses for the noble gases. The high cost of obtaining krypton has limited its uses. Still, scientists and ordinary people use krypton in many ways that might surprise you. In fact, it might be helping to provide the light you are using to read this book!
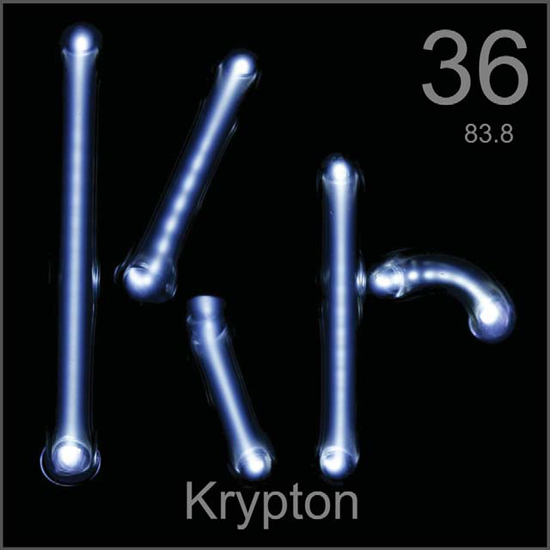
K ryptons chemical symbol is Kr. Its name comes from the Greek word kryptos, which means hidden. Sir William Ramsay and Morris Travers called it that because it was so hard to isolate it from the air. They knew some unknown element was present, but it took many attempts before they were able to separate it from other elements in the air. There is nothing special about the hidden gass appearance. It is colorless, odorless, and tasteless. Krypton looks just like air. Krypton is harmless. The only way it could hurt you is if you were to breathe pure krypton and nothing else. Even then, it is not really the krypton that hurts youit is the lack of oxygen.
Scientists have discovered krypton is useful in many ways. It is used in lighting, lasers, and medicine. It is used to detect leaks in sealed containers and measure X-rays. Scientists have even used it to determine the age of water locked away in spaces deep underground!
The History of Krypton
Krypton was discovered in 1898. However, the story of its discovery actually began more than a century earlier.
In 1785, British chemist Henry Cavendish was studying the formation of nitrous oxide (N2O) by passing a spark through a mixture of oxygen (O2) and air, which is mostly nitrogen. He noticed that each time he did this, there was always a little chemically nonreactive gas left over. Unfortunately, Cavendish did not do what later scientists might have done. He made no effort to study this nonreactive gas. Neither he nor anyone else realized it, but this was the first evidence of the noble gases.

Sir William Ramsay is shown here in his University College laboratory around 1900. He was knighted for his work in 1902 and received the Nobel Prize in Chemistry two years later.
More than a century later, British physicist Lord Rayleigh (John William Strutt) discovered that nitrogen he extracted from ammonia (NH3) was less dense than nitrogen he isolated from air. This surprised him.
Rayleigh theorized that the nitrogen isolated from ammonia was combined with a lighter gas, and this was what made it less dense than nitrogen isolated from air.
Sir William Ramsay thought the reverse was true. He thought the nitrogen obtained from air was combined with a heavier gas. Ramsay suggested this to Rayleigh, and both men began experiments to isolate this heavier gas. They worked separately but exchanged letters almost daily. Finally, in 1894, they both succeeded in isolating the heavier gas. Since it was nonreactive, they named it argon (Ar), from the Greek word argos, which means the lazy one.
In 1895, Ramsay was trying to find sources of argon in minerals when he discovered helium (He). Helium had previously been known only as an element in the sun and had been believed to be a metal. Ramsays discovery demonstrated that helium was actually a nonreactive gas.

Sir William Ramsay used this spectroscope in his work on the noble gases. A spectroscope separates the light given off by an element into its different colors so that it can be studied.
Ramsay believed argon and helium belonged to a previously unknown group of elements. He also believed there were other elements in the group and set out to find them. In 1898, Ramsay and his assistant, Morris Travers, discovered three new nonreactive gases. One was krypton. Ramsay named the second new gas neon (Ne), from the Greek word neos, which means new. He named the third new gas xenon (Xe), from the Greek word xenos, which means strange. For his work, Ramsay received the Nobel Prize in Chemistry in 1904.
William Ramsay, University Professor