Mahrwald - Modern Methods in Stereoselective Aldol Reactions
Here you can read online Mahrwald - Modern Methods in Stereoselective Aldol Reactions full text of the book (entire story) in english for free. Download pdf and epub, get meaning, cover and reviews about this ebook. year: 2013, publisher: Wiley, genre: Romance novel. Description of the work, (preface) as well as reviews are available. Best literature library LitArk.com created for fans of good reading and offers a wide selection of genres:
Romance novel
Science fiction
Adventure
Detective
Science
History
Home and family
Prose
Art
Politics
Computer
Non-fiction
Religion
Business
Children
Humor
Choose a favorite category and find really read worthwhile books. Enjoy immersion in the world of imagination, feel the emotions of the characters or learn something new for yourself, make an fascinating discovery.
- Book:Modern Methods in Stereoselective Aldol Reactions
- Author:
- Publisher:Wiley
- Genre:
- Year:2013
- Rating:4 / 5
- Favourites:Add to favourites
- Your mark:
- 80
- 1
- 2
- 3
- 4
- 5
Modern Methods in Stereoselective Aldol Reactions: summary, description and annotation
We offer to read an annotation, description, summary or preface (depends on what the author of the book "Modern Methods in Stereoselective Aldol Reactions" wrote himself). If you haven't found the necessary information about the book — write in the comments, we will try to find it.
Mahrwald: author's other books
Who wrote Modern Methods in Stereoselective Aldol Reactions? Find out the surname, the name of the author of the book and a list of all author's works by series.
Modern Methods in Stereoselective Aldol Reactions — read online for free the complete book (whole text) full work
Below is the text of the book, divided by pages. System saving the place of the last page read, allows you to conveniently read the book "Modern Methods in Stereoselective Aldol Reactions" online for free, without having to search again every time where you left off. Put a bookmark, and you can go to the page where you finished reading at any time.
Font size:
Interval:
Bookmark:

Related Titles
Majumdar, K. C., Chattopadhyay, S. K. (eds.)
Heterocycles in Natural Product Synthesis
2011
ISBN: 978-3-527-32706-5
Poupon, E., Nay, B. (eds.)
Biomimetic Organic Synthesis
2011
ISBN: 978-3-527-32580-1
Nicolaou, K. C., Chen, J. S.
Classics in Total Synthesis III
Further Targets, Strategies, Methods
2011
ISBN: 978-3-527-32958-8
Yang, J
Six-Membered Transition States in Organic Synthesis
2010
ISBN: 978-0-470-65258-9
Joule, J. A., Mills, K.
Heterocyclic Chemistry
2010
ISBN: 978-0-470-65258-9
Zabicky, J. (ed.)
The Chemistry of Metal Enolates
2009
ISBN: 978-0-470-06168-8
Carreira, E. M., Kvaerno, L.
Classics in Stereoselective Synthesis
2009
ISBN: 978-3-527-32452-1
The Editor
Prof. Dr. Rainer Mahrwald
Humboldt-Universitt Berlin
Institut fr Chemie
Brook-Taylor-Str. 2
12489
Berlin
All books published by Wiley-VCH are carefully produced. Nevertheless, authors, editors, and publisher do not warrant the information contained in these books, including this book, to be free of errors. Readers are advised to keep in mind that statements, data, illustrations, procedural details or other items may inadvertently be inaccurate.
Library of Congress Card No.: applied for
British Library Cataloguing-in-Publication Data
A catalogue record for this book is available from the British Library.
Bibliographic information published by the Deutsche Nationalbibliothek
The Deutsche Nationalbibliothek lists this publication in the Deutsche Nationalbibliografie; detailed bibliographic data are available on the Internet at .
2013 Wiley-VCH Verlag GmbH & Co. KGaA, Boschstr. 12, 69469 Weinheim, Germany
All rights reserved (including those of translation into other languages). No part of this book may be reproduced in any form by photoprinting, microfilm, or any other means nor transmitted or translated into a machine language without written permission from the publishers. Registered names, trademarks, etc. used in this book, even when not specifically marked as such, are not to be considered unprotected by law.
Print ISBN: 978-3-527-33205-2
ePDF ISBN: 978-3-527-65674-5
ePub ISBN: 978-3-527-65673-8
oBook ISBN: 978-3-527-65671-4
mobi ISBN: 978-3-527-65672-1
Cover Design Adam-Design, Weinheima
Typesetting Laserwords Private Limited, Chennai, India
Preface
Stereoselectivity is one of the most important aspects for natural product chemists. Following the increasing possibility of detection and assignment of stereogenic centers, a tremendous increase in stereoselective methods of organic reactions, particularly aldol reactions, has been noticed. In the beginning of this development, only sporadic examples of stereoselective aldol reactions were described, mostly in the context of total syntheses of natural products. An outstanding early example is the R. B. Woodward's proline-catalyzed aldol addition in the total synthesis of erythronolide A at the Harvard University in 1981. In the following three decades, a vast arsenal of stereoselective aldol additions has been developed (see Figure).
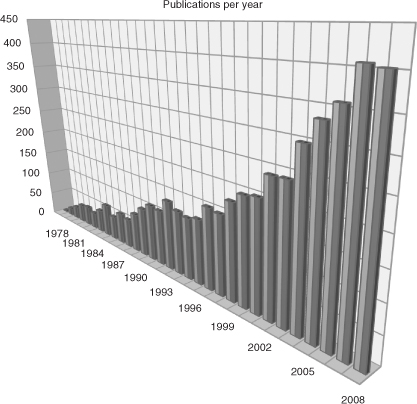
This book provides a comprehensive review of modern aldol reactions, especially in the aspect of how to achieve high stereoselectivity diastereoselectivity as well as enantioselectivity. Stereoselection is discussed under several different aspects. One aspect is the deployment of different substrates acetate or propionate aldol reactions. Another aspect is the mode of action including metal enolate chemistry, Lewis acid as well as Lewis base catalysis, enzymatic catalysis, and organocatalysis. There are some overlappings of these aspects in the chapters covering the cross-cutting themes of vinyloguos Mukaiyama reaction or asymmetric inductions (e.g., compare Scheme ). These overlappings, however, are intentional in order to give a comprehensive insight into the techniques for installing required configurations during aldol reactions. The utility of the corresponding methods is shown in the context of total syntheses of natural products. All chapters are thoroughly well written by experts in the respective fields.
It is my pleasure to express profound gratitude to the 15 authors for their huge endeavor to organize and summarize this vast amount of material. It has been a great pleasure for me to work with this team of authors at all times. Finally, my special thanks go to Elke Maase and Bernadette Gmeiner at WILEY for their fine work in making this book a reality.
Berlin, Autumn 2012
Rainer Mahrwald
List of Contributors
Font size:
Interval:
Bookmark:
Similar books «Modern Methods in Stereoselective Aldol Reactions»
Look at similar books to Modern Methods in Stereoselective Aldol Reactions. We have selected literature similar in name and meaning in the hope of providing readers with more options to find new, interesting, not yet read works.
Discussion, reviews of the book Modern Methods in Stereoselective Aldol Reactions and just readers' own opinions. Leave your comments, write what you think about the work, its meaning or the main characters. Specify what exactly you liked and what you didn't like, and why you think so.










