1. Purple Haze: Introducing Our Guide
Outside of Chicagos City Hall is a giant Picasso sculpture of a weeping woman. For the more artistically challenged, it takes quite a while before you can see it, before you can really make out what Picasso was getting at and how he got there. Five miles to the south of City Hall, in the basement of the University of Chicagos Chemistry Department, lies a piece of glassware of which the great artist would have been proud.
Again to the uninitiated, it takes quite a while to see it. It looks like a deranged spider; indeed, those who work with it call it the Tarantula. When it is working in the darkened laboratory in which it sits, it is suffused by a purple haze and resonates to an electric hum. The Tarantula is not a work of art in the conventional sense, although it is certainly a tribute to the art of the glassblower who made it. This artistic glassware is a discharge tube, a device for making electrically charged chemicals that are normally only found high up in the atmosphere or in the depths of outer space.
We will be returning to the Tarantula shortly.
The Tarantulas owner is Takeshi (just call me) Oka, (now Emeritus) Professor of Chemistry and Astronomy, graduate of the University of Tokyo, distinguished member of the British and the Canadian Royal Societies, holder of many other distinctions from a scientific career that now spans six decades (Figure ). In Chicago, Oka runs the Oka Ion Factory , a laboratory that has paved the way in the study of chemicals that are called molecular ions.
Ions derive their name from the Greek ion , meaning moving thing, and they were given this name by Michael Faraday, Professor of Chemistry at the Royal Institution in London between the years of 1833 and his death in 1867. Ions, explained Faraday, are what move in a chemical solution, or in a more modern application a fluorescent light tube, when you run an electric current through it. Opposites attract cations are positively charged, and travel towards the negatively charged cathode. Conversely anions are negatively charged and head for the you guessed it positively charged anode.
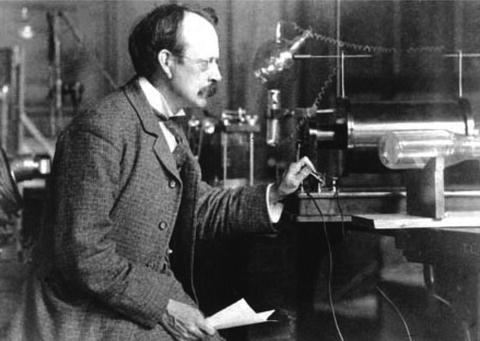
The smallest element of negative charge is called the electron, the first sub-atomic particle ever discovered in 1897 by the British physicist Joseph John (J.J.) Thomson (Figure ). Atoms are made up of electrons surrounding a nucleus, positively charged protons and electrically neutral neutrons. Atoms may become positively charged by dumping a negatively charged electron; and they then become cations like the Sodium atom in common table salt. Or atoms may become negatively charged by picking up an electron and then become anions like the Chlorine atom in the same salt crystal.
Molecules are groups of atoms more or less tightly held together, like Water. In Water, two Hydrogen atoms combine with one Oxygen atom to form the Water molecule. Molecular ions are electrically charged molecules that have either been careless with their electrons molecular cations or greedy for them molecular anions. Molecular ions are literally everywhere, and even in Water, that benign prerequisite of life as we know it, one molecule in ten million has had enough of neutrality and become a cation. And, to maintain electrical balance, one has become an anion; therefore scientists find molecular ions fascinating.
Oka with his Ion Factory is to molecular ions what Henry Ford was to automobiles. (The Ion Factory could also have been called the Professor Factory; there is many a university around the world who owes at least one of its Chemistry professors to the training they received at the hands of Oka, and fellowship his lab generated.) But this is not the story of the Oka Ion Factory itself, although we shall return to it again in our story. Our adventure goes way beyond the confines of the University of Chicago, far out into space beyond our galaxy, the Milky Way, and far back in time to an era in which very few of the chemicals that make up our world had been formed. On our adventure, we shall follow the fortunes of a tiny triangular adventurer, so small that ten billion of them standing in line stretch for little more than a meter.
Our guide is a molecular ion that goes by the name of H3+ (read H-three-plus, if you want to). So what, exactly, is H3+ you may ask?
For starters there is a big clue or two in the name. All elements have a chemical sign to indicate their atoms H for Hydrogen, He for Helium, C for Carbon, N for Nitrogen, O for Oxygen, Cl for Chlorine, etc. So you can see that the chemical signs are either one or two letters long. When atoms combine to form a molecule, the molecule gets its own chemical symbol, known as a formula, derived from the atoms that make it up. The formula for common salt is NaCl, which shows that it is made up of equal numbers of Sodium (Na for the Latin word, Natrium) and Chlorine (Cl) atoms. The formula of Water, H2O, indicates Hydrogen atoms combining with Oxygen in the ratio of two-to-one.
Figure 1.1
Takeshi Oka at work in his laboratory at the University of Chicago: credit Oka Ion Factory, University of Chicago.
Figure 1.2
J.J. Thomson giving a lecture demonstration in the Cavendish Laboratory at the University of Cambridge: credit The Cavendish Laboratory, University of Cambridge.
But H3+ only has H in it; there are no other atoms in it. In an exclusive fashion, in H3+, Hydrogen has decided simply to combine with itself, and it turns out that this is not so unusual. Oxygen atoms like to hang around in pairs, if there is no better offer at hand, to form O2 molecules, the stuff of air that we take in through the walls of our lungs to keep us alive. Nitrogen and Chlorine atoms will also happily keep each other company, as N2 and Cl2. And Hydrogen is most often found doubled up as the molecule H2.
Nor is three necessarily a crowd. Oxygen atoms will hold hands with two others to form Ozone, O3, a pollutant at street level but a life saver high in the Earths atmosphere where it blocks out harmful ultraviolet radiation. Indeed, if it were not for Oxygen tripling up in the form of Ozone, life on Earth would be impossible today. And there are many atoms that will form huge conglomerates. Pure Carbon is the most prolific of them all; it forms endless chains in graphite, extensive crystals in diamond and ball-shaped clusters of C60 60 atoms of Carbon joined together in the form of a miniature soccer ball and even bigger.
So we should not be surprised at three Hydrogens hanging out together, although as we will see later it actually was a surprise when it was first discovered.