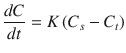Springer International Publishing AG 2018
Marzena Rams-Baron , Renata Jachowicz , Elena Boldyreva , Deliang Zhou , Witold Jamroz and Marian Paluch Amorphous Drugs
1. Why Amorphous Drugs?
Low aqueous solubility of active pharmaceutical ingredients (APIs ) is one of the most important challenges facing drug development researchers today [].
The rate of drug absorption depends on the complex interplay of various physicochemical and physiological conditions [].
The increasing amount of poorly water soluble chemical entities appearing during development research motivates pharmaceutical companies to search for novel solubilizing approaches able to overcome the urgent problem of their inefficient biopharmaceutical performance []. Increasing the dose may induce the desired outcome, however, it raises other problems relating to the proper patient compliance.
Although the nature of solubility and dissolution process are different, the former is a purely thermodynamic phenomenon while the latter is a kinetic event, they are closely related to each other. This relationship can be rationalized by modified Noyes-Whitney equation []:
where dC / dt is the dissolution rate , C s is the drug solubility at saturated equilibrium condition and C t denotes the concentration of drug dissolved at time t . The constant K = AD / h depends on the diffusion coefficient D , value of surface area available for dissolution A and the thickness of diffusion layer h . Various physicochemical and structural factors may tune the parameters in Eq. ( ].
Table 1.1
Formulation approaches on the basis of BCS classification
BCS class | I | II | III | IV |
|---|
Solubility | High | Low | High | Low |
Permeability | High | High | Low | Low |
Examples | Verapamil hydrochloride, warfarin sodium, | Diazepam, ibuprofen, glibenclamide, nevirapine, nifedipine, ritonavir | Cimetidine, amoxicillin, captopril, chloramphe-nicol | Dapsone, paracetamol, sulfamethoxazole |
Formulation strategy | Capsule or tablet | Physical modifications: particle size reduction solid state modifications (polymorphs, co- crystals , amorphous forms) complexation solubilization by surfactants drug dispersion in appropriate carrier Chemical modifications: prodrag application salt formation | Capsule or tablet, absorption enhancers | The same as for BCS II, absorption enhancers |
Adopted from []
Among the available approaches aimed at improving the dissolution behavior of poorly- water soluble drugs amorphization has been considered. Conversion of crystalline drugs into the amorphous form has been recognized as an effective way to achieve the longstanding goal of pharmaceutical science and drug developments, i.e. beneficial drug dissolution in vivo [].
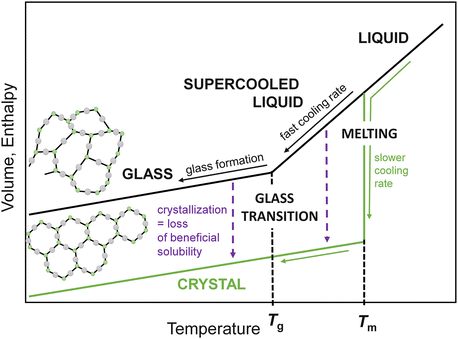
The differences between crystalline and amorphous solids are schematically depicted in Fig. ]. From a pharmaceutical perspective both glassy and supercooled liquid states are relevant. Usually, we keep the drugs at room temperature which corresponds to the glassy state of most pharmaceuticals. However, it is necessary to study amorphous drugs both below and above Tg since higher-temperature conditions corresponding to the supercooled liquid state may be applied during drug manufacturing. Due to higher molecular mobility the risk of drug conversion to crystalline form increases.
Fig. 1.1
Temperature dependence of volume and enthalpy at constant pressure. Fast cooling may lead to glass formation, while for slower cooling rates the crystallization may likely occur. Besides, crystallization may be observed from glassy or supercooled liquid states canceling any improvements in drug dissolution properties
In general, the proper processing of crystalline material (e.g. by mechanical activation during milling , fast melt cooling, rapid precipitation from solution) []. Based on simple thermodynamic considerations they estimated that in the case of the amorphous forms 10- to 1600-fold improvement of drug water solubility in comparison to crystals should be expected. However, the measured values are usually significantly lower which was explained by difficulties in their experimental determination.
The excess thermodynamic properties of the amorphous state , like its greater entropy, enthalpy and free energy as well as its higher molecular mobility make the amorphous drugs more prone to crystallization. So far finding an effective stabilization approach is the major challenge related to the development of drugs in the amorphous form. The crystalline drug forms, more stable and easier to handle, dominate the pharmaceutical market for practical and economical reasons. However, the importance of the problem of insufficient APIs solubility encourages pharmaceutical companies to support and invest in new solutions, even those requiring additional efforts to obtain the beneficial drug absorption in vivo. Therefore, drug compositions based on amorphous active ingredients attract particular interest despite their unstable and problematic nature. The emphasis in developing amorphous formulations is put on searching drug compositions providing stability at each stage of drug processingfrom its manufacturing to administration. The progress we are witnessing today, reflected in the growing number of amorphous products available on the market is related to the successful implementation of amorphous solid dispersions technology. This concept, substantially improving water solubility and effectively protecting against drug recrystallization , allows for successfully entering of amorphous products onto the pharmaceutical market and secured their stable position in the offer of pharmaceutical companies.
Searching for stable amorphous drug formulations is a complex issue which cover a variety of challenges that we have to face at each stage of the drug product lifecycle. All should be predicted and resolved at the initial stage of research and drug development. Without complete understanding of the theoretical principles which are responsible for the recrystallization behavior, achieving the desired goal of producing efficient, well-soluble and safe amorphous drug product will be unattainable. It is well known that the process of drug discovery is extremely costly and highly risky. To save time and money unnecessarily lost during verification of ineffective solutions, a rational approach to the problem of amorphous drug instability is required. Such an approach requires interdisciplinary knowledge, skills and insights into the problems. The recrystallization of amorphous content might be promoted by elevated temperature, mechanical stress or humidity at each stage of drug processing, storage or even administration. The systematic investigations of crystallization behavior of amorphous drugs at different thermodynamic conditions and a comprehensive insight into manufacturing procedures allows one to establish processing conditions minimizing the risk of drug recrystallization . Only in-depth understanding of factors controlling crystallization kinetics allows for design of effective stabilizing solutions. The lack of such knowledge makes it impossible to understand the reasons of unexpected failure at a formulation stage.