Lithium batteries have existed in various forms since the 1970s, and innovations in the 80s and 90s have led to the familiar lithium battery cells that we know today. Current research on lithium batteries has produced battery cells capable of extreme performance, for example, 100% recharging in just a few seconds. However, these current advances are strictly experimental and wont see commercialization for many years, potentially decades. The information in this book covers the types of lithium batteries that are commercially available today and will likely remain available well into the future.
Uses for lithium batteries
Today, lithium batteries are used for a seemingly endless number of applications. They can be found everywhere from electric vehicles to NASAs spacesuits. Due to their lightweight and energy dense properties, lithium batteries are perfect for an incredibly wide range of applications.
In the past, lithium batteries were mostly used by original equipment manufacturers (OEMs) for use in consumer products. These big manufacturers built lithium batteries suited to their needs for specific products or large clients. If a hobbyist wanted a battery size or shape that didnt exist, he or she was out of luck. However, today there are many lithium batteries and cells that are readily available directly to consumers for use in, well, whatever we want!
I was introduced to the world of custom lithium batteries during my time spent working in the do-it-yourself (DIY) electric bicycle industry. I have been building lithium batteries for electric bicycles for years, largely because the number and variety of lithium batteries available to the consumer market has always been frustratingly small. If I wanted a specific size battery pack but it didnt already exist, I had no other choice than to make it myself. This opened up a whole new world to me. Suddenly, I could build batteries of any voltage, any capacity and most importantly, any size and shape that I wanted.
But DIY lithium batteries arent only limited to the world of electric bicycles. There are thousands of applications for DIY lithium batteries!
Even though electric cars are becoming increasingly available in the consumer market, it can still be cheaper (and more fun) to build your own. Many people convert all kinds of vehicles into electric vehicles, and they need batteries to do it. Unless you want to buy an expensive, purpose built electric car battery, youll need to know how to assemble your own large battery pack from lithium battery cells.
Just like electric vehicles, home batteries are also becoming increasingly popular. A lithium battery in the back of your closet or hidden in your garage can power a house for days in the event of a power outage. They are also great for storing energy that has been generated on site, such as from solar panels or wind turbines. Home battery storage systems like the Tesla Powerwall are great OEM products, but you can still build your own custom system suited for your unique needs. All you need is the battery know-how!
Drones, wearables, backup batteries, toys, robotics and countless other applications are all ripe for custom DIY lithium batteries. This book will teach you how to design and build lithium batteries for all of these uses and more. Prepare yourself, because by the time you finish this book, you are going to be full of knowledge and rearing to go out and power the world!
How lithium battery cells work
Despite undergoing years of research and development, the electrical and chemical processes that allow lithium battery cells to function is actually fairly simple. As lithium ion batteries are by far the most common form of lithium battery cells, well take a look at how a typical cell works here.
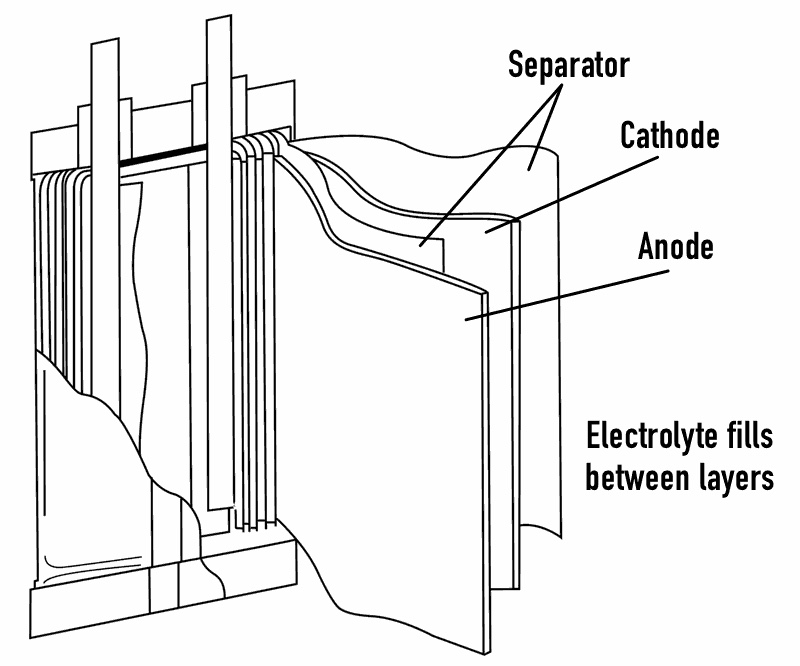
A lithium-ion cell is composed of four main parts:
- Cathode (or positive terminal)
- Anode (or negative terminal)
- Electrolyte
- Porous separator
The cathode varies between different types of cells but is always a lithium compound mixed with other materials. The anode is almost always graphite, and sometimes includes trace amounts of other elements. The electrolyte is generally an organic compound containing lithium salts to transfer lithium ions. The porous separator allows lithium ions to pass through itself while still separating the anode and cathode within the cell.
When the cell is discharged, lithium ions move from the anode to the cathode by passing through the electrolyte. This discharges electrons on the anode side, powering the circuit and ultimately any device connected to the circuit. This process is demonstrated in the diagram below. When the cell is recharged, this process is reversed and the lithium ions pass back from the cathode to the anode, which is opposite to the diagram below.
The actual process is quite simple. The major differences (and where things get more complicated) are in the shape of the cells and their slight chemical changes. Well cover all of that information in the next two chapters.
Chapter 2: Form factors of lithium cells
Lithium battery cells are available in a number of different form factors, yet their underlying construction is always the same. All lithium battery cells have a positive electrode (cathode), a negative electrode (anode), an electrolyte material and some type of porous separator in between that allows lithium ions to move between the cathode and the anode. Well talk about how changes in the chemistry of different li-ion cells can affect them in the next chapter. For now, the main difference between various shapes of lithium cells is the way they are assembled.
Pouch cells
Pouch cells are the simplest form of lithium battery cells. They look like a tin foil bag (or pouch, get it?) and have two terminals at an edge of the pouch. Inside the pouch is a cathode and anode on opposite sides separated by the porous separator and with the electrolyte on either side of the separator. This cathode-electrolyte-anode sandwich is folded back and forth many times within the pouch to increase the capacity of the battery.












