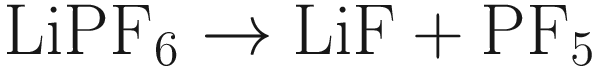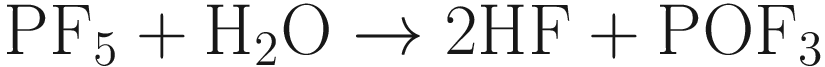SpringerBriefs in Energy
SpringerBriefs in Energy presents concise summaries of cutting-edge research and practical applications in all aspects of Energy. Featuring compact volumes of 50 to 125 pages, the series covers a range of content from professional to academic. Typical topics might include:
A snapshot of a hot or emerging topic
A contextual literature review
A timely report of state-of-the art analytical techniques
An in-depth case study
A presentation of core concepts that students must understand in order to make independent contributions.
Briefs allow authors to present their ideas and readers to absorb them with minimal time investment.
Briefs will be published as part of Springers eBook collection, with millions of users worldwide. In addition, Briefs will be available for individual print and electronic purchase. Briefs are characterized by fast, global electronic dissemination, standard publishing contracts, easy-to-use manuscript preparation and formatting guidelines, and expedited production schedules. We aim for publication 812 weeks after acceptance.
Both solicited and unsolicited manuscripts are considered for publication in this series. Briefs can also arise from the scale up of a planned chapter. Instead of simply contributing to an edited volume, the author gets an authored book with the space necessary to provide more data, fundamentals and background on the subject, methodology, future outlook, etc.
SpringerBriefs in Energy contains a distinct subseries focusing on Energy Analysis and edited by Charles Hall, State University of New York. Books for this subseries will emphasize quantitative accounting of energy use and availability, including the potential and limitations of new technologies in terms of energy returned on energy invested.
More information about this series at https://link.springer.com/bookseries/8903
Futoshi Matsumoto and Takao Gunji
Water in Lithium-Ion Batteries
Logo of the publisher
Futoshi Matsumoto
Department of Materials and Life Chemistry, Kanagawa University, Yokohama, Japan
Takao Gunji
Department of Materials and Life Chemistry, Kanagawa University, Yokohama, Japan
ISSN 2191-5520 e-ISSN 2191-5539
SpringerBriefs in Energy
ISBN 978-981-16-8785-3 e-ISBN 978-981-16-8786-0
https://doi.org/10.1007/978-981-16-8786-0
The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd. 2022
This work is subject to copyright. All rights are solely and exclusively licensed by the Publisher, whether the whole or part of the material is concerned, specifically the rights of translation, reprinting, reuse of illustrations, recitation, broadcasting, reproduction on microfilms or in any other physical way, and transmission or information storage and retrieval, electronic adaptation, computer software, or by similar or dissimilar methodology now known or hereafter developed.
The use of general descriptive names, registered names, trademarks, service marks, etc. in this publication does not imply, even in the absence of a specific statement, that such names are exempt from the relevant protective laws and regulations and therefore free for general use.
The publisher, the authors and the editors are safe to assume that the advice and information in this book are believed to be true and accurate at the date of publication. Neither the publisher nor the authors or the editors give a warranty, expressed or implied, with respect to the material contained herein or for any errors or omissions that may have been made. The publisher remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This Springer imprint is published by the registered company Springer Nature Singapore Pte Ltd.
The registered company address is: 152 Beach Road, #21-01/04 Gateway East, Singapore 189721, Singapore
Preface
Water (H2O) in lithium-ion batteries (LIBs), which are constructed with anodes, cathodes and organic electrolytes that contain lithium salts, can degrade the cell performance and seriously damage the materials. However, because a small amount of H2O in cells contributes to the formation of a solid electrolyte interphase (SEI), the complete removal of H2O from cells lowers battery performance and increases the expense of H2O removal from the battery materials. The optimal concentration of H2O for each battery material has been determined, and these concentrations are maintained with appropriate removal methods and H2O scavengers that were recently developed to establish both high performance and low cost. More recently, to achieve both the safety and low cost of LIBs, the development of anode and cathode preparations by aqueous processes and aqueous LIBs in which aqueous electrolytes containing lithium salts are used as electrolytes has progressed. In this review, information on the H2O content in LIBs, the reactivity of anodes, cathodes and electrolytes with water and the processes underlying H2O resistance in LIB materials is reviewed from the perspective of H2O concentration and LIB stability. The goal of this review is to provide appropriate information concerning the amount of H2O needed in cells to achieve stable and high cell performance.
Futoshi Matsumoto
Takao Gunji
Yokohama, Japan
Contents
The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd. 2022
F. Matsumoto, T. Gunji Water in Lithium-Ion Batteries SpringerBriefs in Energy https://doi.org/10.1007/978-981-16-8786-0_1
1. Introduction
Futoshi Matsumoto
(1)
Department of Material and Life Chemistry, Kanagawa University, Yokohama, Japan
Futoshi Matsumoto (Corresponding author)
Email:
Abstract
In this chapter, history of lithium-ion batteries (LIBs) and their problems which should be resolved in future are overviewed. In addition, the problem caused by water which is main part of this book is raised with the reaction occurred with water in LIBs. Finally, the purpose and the content of this book is explained.
Keywords
Water-resistant properties Moisture Lithium salt Hydrogen fluoride Solid-state electrolytes Rechargeable aqueous batteries
In the thirty years since Sony successfully commercialized LIBs in 1991, they have achieved remarkable progress in the area of portable devices, in which these batteries are used as power sources [].
Among the currently commercially available LIBs, invasion of H2O into batteries causes the degradation of battery performance [].