FIGURE 21-2 (see )
The Kodachrome process of color photography.
GENERAL
CHEMISTRY
Copyright 1947, 1950, 1970 by Linus Pauling. All rights reserved.
This Dover edition, first published in 1988, is an unabridged, slightly altered and corrected republication of the work published by W. H. Freeman and Company, San Francisco in 1970. The endpaper tables have been moved to the front section of the book and the color illustrations have been moved to the inside covers.
Library of Congress Cataloging-in-Publication Data
Pauling, Linus, 1901
General chemistry / Linus Pauling.
p. cm.
Reprint. Originally published: 3rd ed. San Francisco : W.H. Freeman, 1970.
Bibliography: p.
Includes index.
International Standard Book Number
eISBN-13: 978-0-486-13465-9
1. Chemistry. I. Title.
QD33.P34 1988
540dc19 87-32165
CIP
Manufactured in the United States by Courier Corporation
65622517
www.doverpublications.com
Preface
In the first edition of this book, published 22 years ago, I attempted to simplify the teaching of general chemistry by the correlation of the facts of descriptive chemistry, the observed properties of substances, to as great an extent as possible with theoretical principles, especially the theory of atomic and molecular structure. This correlation with theory was extended in the second edition, and is extended still further in the present edition.
The theories of greatest value in modern chemistry are the theories of atomic and molecular structure, quantum mechanics, statistical mechanics, and thermodynamics. In this book I have tried to present in a sound and logical way the development of these theories, in their relation to chemistry. The principles of quantum mechanics are discussed on the basis of the de Broglie wavelength of the electron. The quantized energy levels of a particle in a box are derived by means of a simple assumption about the relation of the de Broglie waves to the walls of the box. No attempt is made to solve the Schrdinger wave equation for other systems, but the wave functions of hydrogen-like electrons are presented and discussed in some detail, and the quantum states for other systems are also discussed.
I have found that an understanding of statistical mechanics (especially in its quantum-mechanical form) is more easily obtained by the beginning student than an understanding of chemical thermodynamics. It is for this reason that I have introduced statistical mechanics before thermodynamics, and have based the discussion of thermodynamics on it.
. It has been customary for many years to include some parts of chemical thermodynamics, especially in relation to chemical equilibrium, in general chemistry, without relating the equations to the basic principles of thermodynamics and statistical mechanics. I think that it will be helpful to the student to have available in his textbook a discussion of the basic theory, even though it may not be possible for him to master the material in the time during which the course is presented.
The amount of descriptive chemistry has been decreased somewhat in this edition, and the presentation of this subject, especially in relation to the nonmetals, has been revised in such a way as to permit greater correlation with the electronic structure of atoms, especially electronegativity. It is not possible to add new material to the one-year course of general chemistry without omitting some of the old material. It is important for the student to know some of the facts of descriptive chemistry, and I have attempted to make a reasonable decision about the extent to which the elimination of descriptive chemistry should be carried out.
There is, of course, somewhat more material in this book than can be mastered by even a very good student in one year. I have assumed that some of the chapters and sections will be omitted in formal study. The inclusion in the book of this extra material makes it available for the clarification of questions that might arise and for additional reading by the interested student.
In this book extensive use is made of the International System of units. Many students have already become acquainted with this system (or the closely related MKS system) through their study of physics. The acceptance of the IS system by most countries in the world and the inherent advantages of the system (the elimination of arbitrary conversion factors) seem to me to justify the transition to the IS units at the present time. The principal change is the use of the joule in place of the calorie as the unit of energy.
This edition, like the preceding editions, is designed especially for use by first-year college university students who plan to major in chemistry or in closely related fields, and by other well-prepared students with a special interest in chemistry. I have assumed that the students have some background of knowledge of physics and mathematics.
I am glad to acknowledge the general assistance of Gustav Albrecht, Barclay Kamb, Peter Pauling, Arthur B. Robinson, and Fred Wall, and also thank John Ricci for preparation of the stereoscopic drawings.
LINUS PAULING
Contents
GENERAL
CHEMISTRY
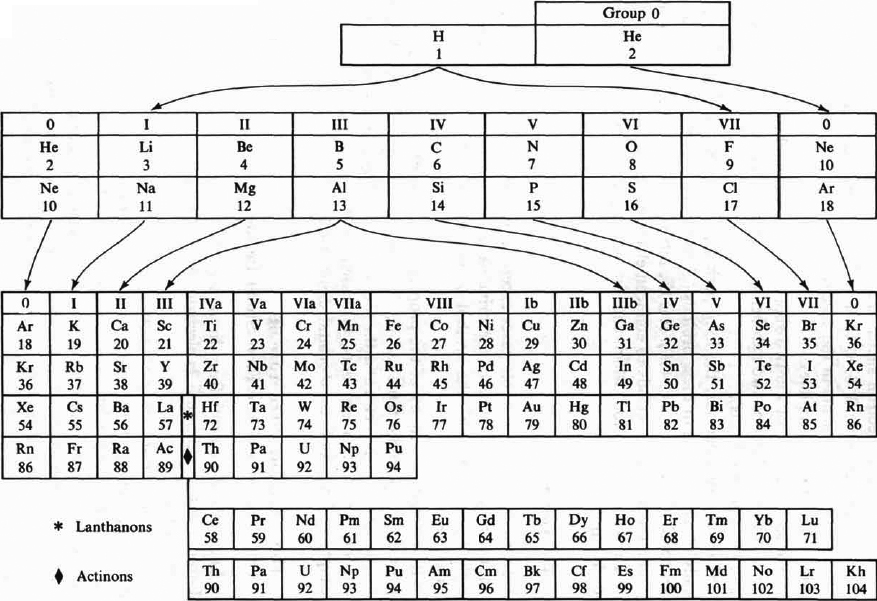
The Periodic System of the Elements
International Atomic Weights
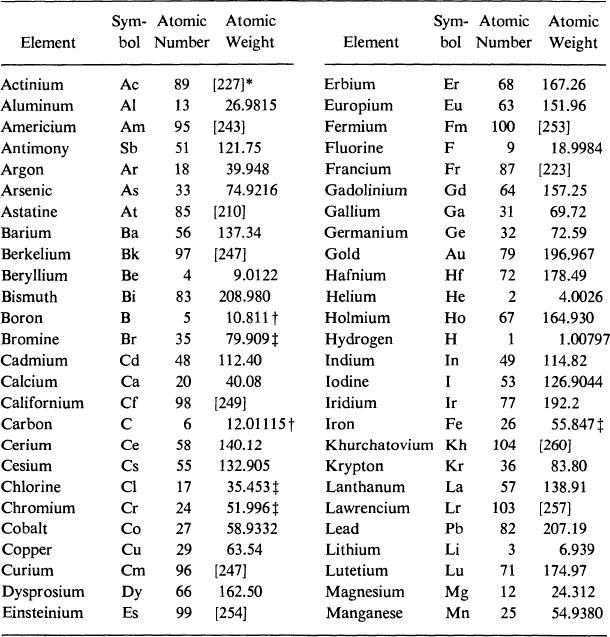
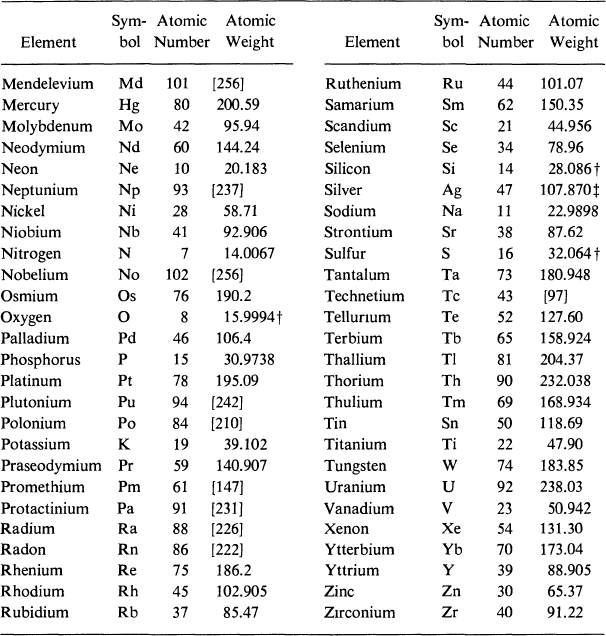
*A value given in brackets is the mass number of the most stable known isotope.
The atomic weight varies because of natural variations in the isotopic composition of the element. The observed ranges are boron, 0.003; carbon, 0.00005; hydrogen, 0.00001; oxygen, 0.0001; silicon, 0.001; sulfur, 0.003.
The atomic weight is believed to have an experimental uncertainty of the following magnitude: bromine, 0.002; chlorine, 0.001; chromium, 0.001; iron, 0.003; silver, 0.003. For other elements the last digit given is believed to be reliable to 0.05.
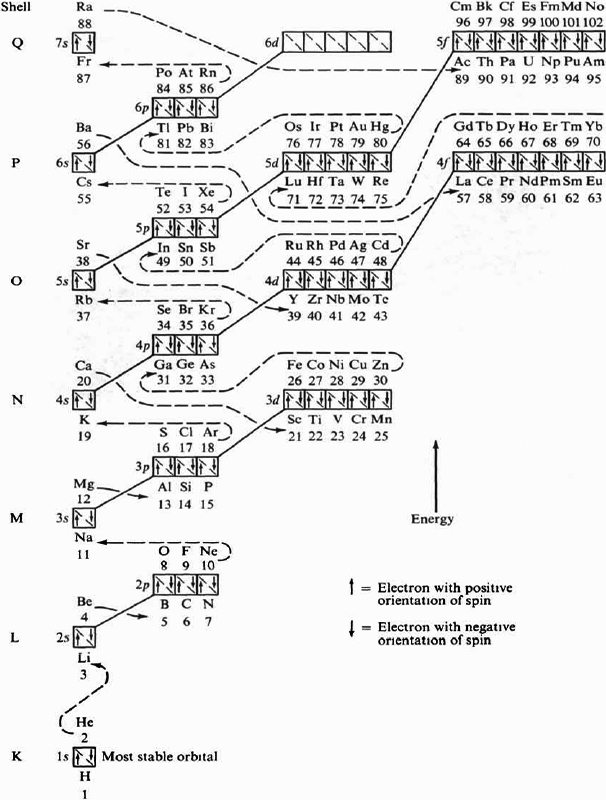
Energy Level Diagram of Electron Shells and Subshells of the Elements
1
The Nature and Properties of Matter
1-1. Matter and Chemistry
The universe is composed of matter and radiant energy. Matter (from the Latin materia, meaning wood or other material) may be defined as any kind of mass-energy (see ) that moves with velocities less than the velocity of light, and radiant energy as any kind of mass-energy that moves with the velocity of light.
The different kinds of matter are called substances. Chemistry is the science of substancestheir structure, their properties, and the reactions that change them into other substances.
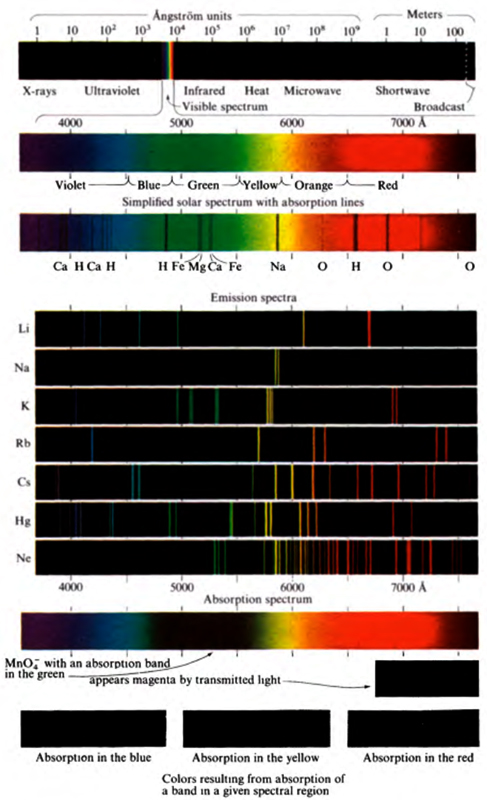
This definition of chemistry is both too narrow and too broad. It is too narrow because the chemist in his study of substances must also study radiant energy, in its interaction with substances. He may be interested in the color of substances, which is produced by the absorption of light. Or he may be interested in the atomic structure of substances, as determined by the diffraction of x-rays () or by the absorption or emission of radiowaves by the substances.
Next page