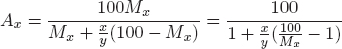CONTENTS

Appendix: Conversion of indices between three and four indices in HCP
Indices based on the three axes, a1 and a2 and c are generally used for hexagonal crystals but are open to the objection that equivalent planes do not have similar indices. For instance, the planes (100) and (10) are both prismatic planes and are equivalent. The same objection applies to the indices of directions. For these reasons the hexagonal indices such as those shown in (same as the figure in footnote on p. 75) are used. In addition to the usual a1 and a2 axes, a third axis a3 is introduced and planes and directions are indicated by (hkil) and [uvtw], respectively. Here, h, k, i or u, v, t are indices corresponding to the a1, a2, and a3 axes. l and w are indices corresponding to the c axis. h, k, i and u, v, t are related by the equations

| (A.1) |
Transformations between the three and four-indices, i.e., between (HKL) and (hkil) and between [UVW] and [uvtw] are given by
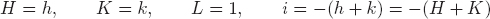
| (A.2) |
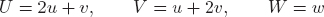

| (A.3) |
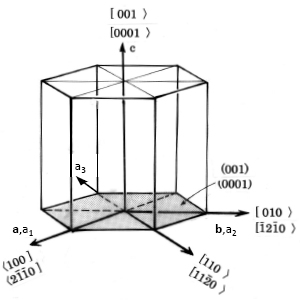
Fig. A.1Coordinates of a, b and c are for three-index system. Coordinates of a1, a2, a3 and c are for four-index system. ( ) stands for plane and [ > or <] stands for orientation.
Chapter 1
Equilibrium Phase Diagrams
1.1General Theory
1.1.1Metallography and phase diagram
Metals are very useful. This is obvious if one looks back on the rise and development of human civilization. It is when human beings, after having experienced the stone age and bronze age, finally reached the iron age that modern civilization flourished. shows the production of steel all over the world. We are living in the middle of the iron age.
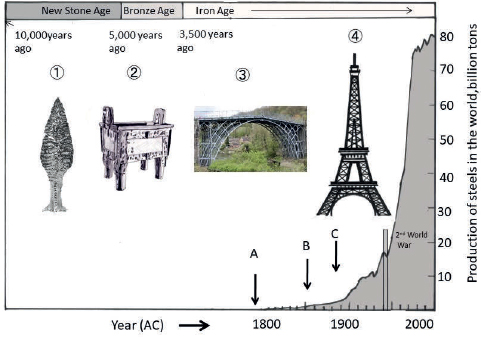
Fig. 1.1History of production of iron (steel).  Dagger in the new stone age.
Dagger in the new stone age.  Bronze in old China (Yin).
Bronze in old China (Yin).  Iron bridge (UK).
Iron bridge (UK).  Eiffel tower (France). A: Iron bridge built (1781). B: Henry Bessemer invented a converter (1856). C: Eiffel tower built (1899).
Eiffel tower (France). A: Iron bridge built (1781). B: Henry Bessemer invented a converter (1856). C: Eiffel tower built (1899).
Metals and alloys are backbones which support modern civilized societies because of their variety and flexibilities. Our ancestors learned diversity of metals and alloys by experience and made most of it, the compilation of which is metallurgy. Metallurgy is now indispensable to control microstructure of not only metallic materials but also ceramics and semiconductors.
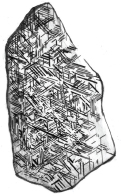
Fig. 1.2Widmannstten pattern.
What is the microstructure of metals and alloys? Till the 18th century metals were not considered as crystals. In the early 19th century it was found that when a meteolite (usually composed of Fe and Ni) was lightly etched with a nitric acid, a pattern such as that shown in appeared. Such a pattern was named Widmansttten pattern after the discoverer Alois von Beckh Widmansttten. This confirmed that metals and alloys are crystals just like minerals. Later this was confirmed by X-ray diffraction.
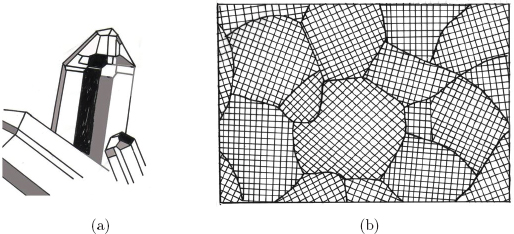
Fig. 1.3(a) Single crystal (quartz). (b) Schematic illustration of a polycrystal.
Many people may think of quartz or diamond when they picture crystals to themselves (composition, it is referred to as a multi-phase alloy. Morphology, size, distribution etc. of aggregates are referred to as microstructure. Properties of metals and alloys depend strongly on their microstructure, and the science to study factors controlling the relationship between microstructure and properties is referred to as metallography. Needless to say, this can be applied to materials in general.
The microstructure depends not only on the kind and the number of components (for instance Fe-Cr or Fe-Cr-Ni) comprising an alloy under consideration but also on its chemical composition (for instance, Fe12%Cr versus Fe18%Cr) and history such as temperature and heat-treatments which the alloy has undergone.
A map to indicate how the microstructure of a particular alloy under investigation depends on temperature is a phase diagram, where the equilibrium states are illustrated as functions of temperature, pressure and chemical composition.
1.1.2Terminology
In metallography and phase diagrams many technical terms are used. Among them, the essential terms necessary to read this book are summarized in the following:
a. Alloy (alloy system)
A mixture that is created by adding other element(s) to a pure metal. The alloying elements can be metal, metalloids and non-metallic elements. Examples are Fe-C alloy (system), Au-Ag-Cu alloy (system) etc. Alloy and system are synonyms.
b. Component
How many kinds of elements are added to make an alloy?
Two-component systembinary system
Three-component systemternary system
More than three-component systemmulti-component system
c. Chemical composition, concentration
Chemical component indicates elements comprising an alloy. Relative amounts of individual elements are referred to as chemical composition or simply composition, usually indicated by percentage, either in weight percent (wt.%, w/o), mass percent (mass %) or atomic percent (at.%, a/o).
Weight concentration indicates the weight of each component (alloying elements) by percentage. Atomic concentration indicates the numbers of atoms by percentage. Needless to say, when one makes an alloy, a weight concentration is used. However, in theoretical calculation an atomic concentration is more convenient.
In the binary system, weight (mass) concentration is converted to atomic concentration using , where Mx, Ax and x stand for weight (mass)%, atomic % and the atomic weight of an element X, respectively. Those corresponding to the other element Y are My, Ay and y.












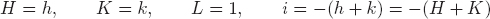
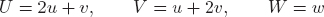



 Dagger in the new stone age.
Dagger in the new stone age.  Bronze in old China (Yin).
Bronze in old China (Yin).  Iron bridge (UK).
Iron bridge (UK).  Eiffel tower (France). A: Iron bridge built (1781). B: Henry Bessemer invented a converter (1856). C: Eiffel tower built (1899).
Eiffel tower (France). A: Iron bridge built (1781). B: Henry Bessemer invented a converter (1856). C: Eiffel tower built (1899).