Mahmoud Gaballa and Carlos A. Ramos
The formed elements of blood include various types of cells or cell fragments, each with different morphology and function. These elements are produced via a process known as hematopoiesis, during which hematopoietic stem cells (HSCs) proliferate and undergo self-renewal or differentiation into lineage-committed progenitors, which continue to differentiate into mature blood cells. This ability to self-renew and the ability to differentiate are two key characteristics of HSCs necessary for normal hematopoiesis (). Self-renewal is the process by which stem cells enter the cell cycle to divide and give rise to more stem cells, thus preserving the stem cell pool. On the other hand, differentiation allows HSCs to develop into more mature cells with progressive lineage commitment. In general, the ability to self-renew diminishes as maturation and lineage commitment progresses.
The capacity of HSCs to differentiate into multiple cell lines is termed multipotency. The hematopoietic hierarchy is a well-orchestrated process that starts with HSCs developing into myeloid and lymphoid progenitor cells. Myeloid progenitor cells continue to develop into erythrocytes, platelets (through fragmentation of megakaryocytes), neutrophils, monocytes, basophils, and eosinophils. In contrast, lymphoid progenitor cells give rise to B lymphocytes, T lymphocytes, natural killer (NK) cells, and a population of dendritic cells (). Thus, a very complex process in the bone marrow gives rise to at least 10 cellular elements on a daily basis. Blood cell numbers are sustained within relatively narrow ranges through this process, with the ability to boost production if needed in states of increased demand. In this chapter, we illustrate our current understanding of hematopoiesis, its stages, the cellular and non-cellular elements involved, and its regulation.
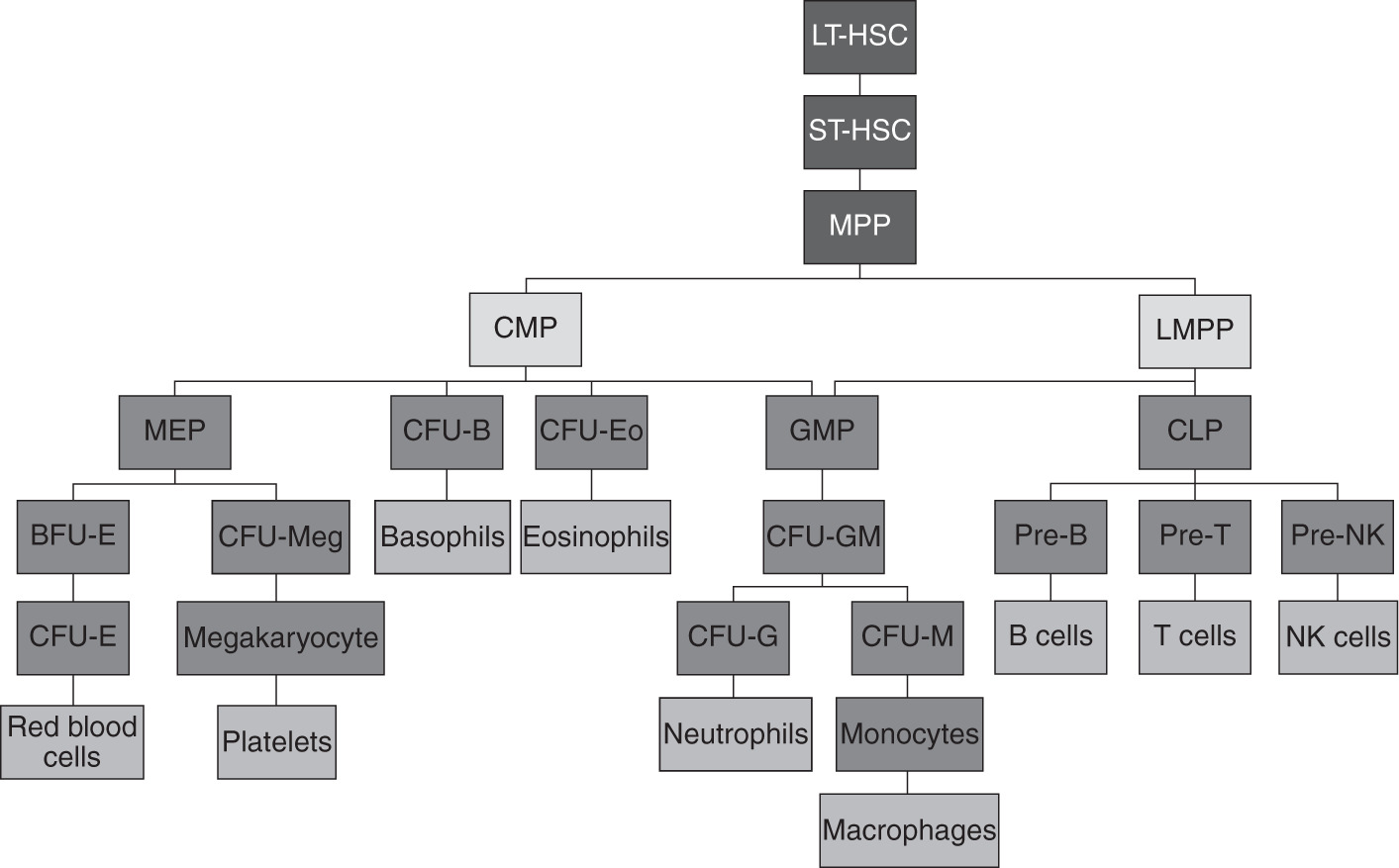
Figure 1.1 Hierarchy of hematopoiesis.
BFU-E, burst-forming unit-erythroid; CFU-B, colony-forming unit-basophil; CFU-E, colony-forming unit-erythrocyte; CFU-Eo, colony-forming unit-eosinophil; CFU-G, colony-forming unit-granulocyte; CFU-GM, colony-forming unit-granulocyte monocyte; CFU-M, colony-forming unit-monocyte; CFU-Meg, colony-forming unit-megakaryocyte; CLP, common lymphoid progenitor; CMP, common myeloid progenitor; GMP, granulocytemacrophage progenitor; LMPP, lymphoid primed multipotent progenitor; LT-HSC, long-term hematopoietic cell; MEP, megakaryocyteerythrocyte progenitor; MPP, hematopoietic multipotent progenitor; NK, natural killer; pre-NK, pre-natural killer; ST-HSC, short-term hematopoietic stem cell.
STAGES AND SITES OF HEMATOPOIESIS
Hematopoiesis starts during embryonic life and continues through fetal, neonatal, and adult life. Embryonic hematopoiesis comprises two waves of events. The first wave, often referred to as primitive hematopoiesis, takes place specifically in the extraembryonic yolk sac, where nucleated primitive erythrocytes are produced. This is a critical step for the development and survival of the embryo since those primitive erythrocytes are the first elements to facilitate oxygen transportation. In addition, myeloid cells produced in the yolk sac subsequently relocate to the central nervous system and skin to develop into microglia and Langerhans cells, respectively (). This process happens once in a lifetime and those cells later continue to develop to form adult HSCs, with the expression of major histocompatibility complex (MHC) class II and cluster of differentiation (CD)45.
In the fetus, hematopoiesis transitions first from the embryonic sites to the liver. At approximately 5 gestational weeks, the HSCs and progenitors travel from the yolk sac or AGM to the liver to establish hematopoiesis ().
At 4 to 5 months of gestation, hematopoiesis begins to occur in the bone marrow, a process termed medullary hematopoiesis, which continues after birth and throughout postnatal life. As we advance into adulthood, hematopoiesis slowly becomes confined to the skull, pelvic bones, vertebrae, and the metaphyseal region of long bones, with the diaphysis of long bones being gradually replaced with adipose tissue. Thus, the active bone marrow of children is proportionally much larger than that of adults, which is thought to occur due to the higher demand for red cells in neonatal and childhood life compared to adulthood. Importantly, in states of medullary insufficiency, as in patients with thalassemia and myelofibrosis, hematopoiesis can revert to its original sites, including the liver and spleen. This is termed extramedullary hematopoiesis. illustrates the sites of hematopoiesis in different stages of life.

Figure 1.2 Sites of hematopoiesis in different stages of life.
HSCs AND THE HEMATOPOIETIC HIERARCHY
The hematopoietic differentiation cascade is akin to an inverted tree, starting with a small pool of HSCs that develop into a larger pool of progenitors, which then further differentiate into many intermediates and finally develop into different mature blood cells. As the cells travel down their hierarchy, they lose the ability to differentiate into other cell lines and progressively become committed to one lineage, that is, lineage-committed progenitors.
HSCs choose early whether to self-renew or to differentiate into more mature cells, with two models proposed to describe this choice, the stochastic (supportive) and the instructive. In the stochastic model, the choice of self-renewal versus differentiation is random. In contrast, in the instructive model, certain microenvironment factors and cytokines directly influence this decision. Notably, cytokines have critical but different roles in both models. In the instructive model, cytokines actively influence the decision, while in the stochastic model, cytokines do not influence the decision but support the survival and proliferation of progenitor cells that eventually differentiate into mature cells (). This issue remains controversial with conflicting data supporting both models.
HSCs are further subclassified as long term (LT-HSCs) and short term (ST-HSCs). The main role of LT-HSCs is self-renewal, and most of their progeny will follow this pathway, while lesser numbers become ST-HSCs ().
Hematopoietic progenitors can be classified into multilineage progenitors and single-lineage progenitors according to their differentiation potential. As illustrated in ). The timing of appearance, the morphology, the cellular components, and the cytokine requirements of the colonies arising in these assays define each of the intermediate forms, with the convention that a cell population identified by a particular assay is referred to by the assay name. CLPs ultimately differentiate into B, T, and NK cells. While myeloid and lymphoid lineages are thought to arise independently, some studies have suggested that the granulocytemacrophage progenitor (GMP) can also be derived from LMP.
Whereas many of the intermediates are not morphologically distinguishable, the majority of the final differentiation steps toward individual lineages can be identified by conventional histochemistry, where a blast form gives rise to more mature forms that progressively resemble the fully differentiated elements. lists the different types of mature blood cells as well as their morphologic characteristics and function.
CLUSTERS OF DIFFERENTIATION
As HSCs and progenitors differentiate, they acquire distinct cell surface antigen markers, also known as CDs, while losing the antigens associated with more primitive cells. CDs can be used to identify stem cells and diverse types of progenitors, intermediates, and mature blood cells of different lineages, even at stages when morphology alone would not be enough for cell identification. Identifying cell subpopulations via CDs is often done through monoclonal antibodies and flow cytometry, with marker panels being readily available commercially. The number of described CDs continues to grow and has exceeded 370 to date. Utilization of CDs in the clinical arena has revolutionized the way we understand, diagnose, classify, and treat hematologic diseases, and is now the standard of care to be incorporated in their diagnostic workup.












