Derek B. Lowe
STERLING and the distinctive Sterling logo are registered trademarks of Sterling Publishing Co., Inc.
Text 2016 by Derek B. Lowe
All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted, in any form or by any means (including electronic, mechanical, photocopying, recording, or otherwise) without prior written permission from the publisher.
For information about custom editions, special sales, and premium and corporate purchases, please contact Sterling Special Sales at 800-805-5489 or specialsales@sterlingpublishing.com.
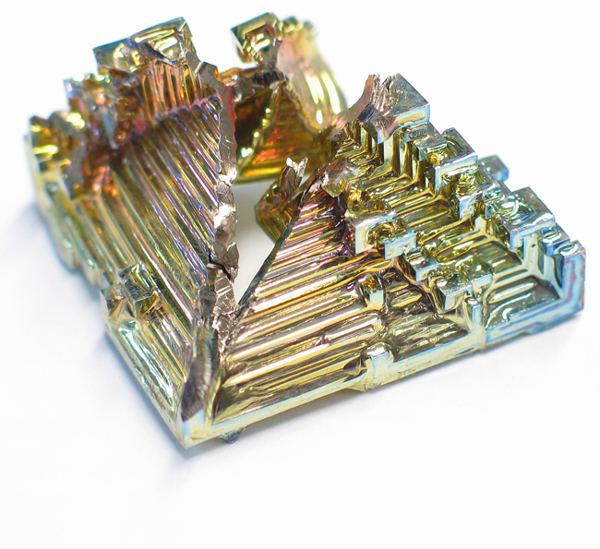
The element bismuth melts easily (like its chemical neighbors, mercury and lead) and forms these characteristic staircase crystals as it cools. The rainbow colors come from a microscopically thin layer of bismuth oxide on the surface.
Introduction
Electrons, protons, and neutrons form atomsthats physics. But atoms bond together to form molecules, and thats chemistry. Beginning textbooks for chemistry students tend to say something about the Central Science to emphasize chemistrys role in scientific progressand in the process make their readers feel better about taking the coursebut that characterization is truly accurate. Chemistry really does occupy the middle ground between physics and biology, claiming territory from each of those sciences as well as its own. A look through this book shows what that means in practice. There are entries that straddle the borderline between physical chemistry and chemical physics, and others that land somewhere between biological chemistry and chemical biology. (And yes, those are all real names for fields of study, although even their own practitioners might disagree about what falls where!)
The study of chemistry is older than human writing. Only archaeologists could tell us when and where the early chemical experiments might have taken place, and the very first stirrings surely left no traces at all. When some distant ancestor of ours wondered about fire and its effects, thought about the colors of rocks and pigments, or ground up plants for medicine, they were making the sorts of chemical explorations that continue today. A modern chemist has connections throughout human history, to Bronze Age metalsmiths and Egyptian priests, to Chinese scholars and Persian alchemists. We can look back on many of these people and remark on all the things that they got wrong, but whats important is what they got right, because that built the science we have now.
Its worth remembering, too, that science itself is a very recent thing. Note the concentration of this books entries on the historical timeline: There is a long, slow buildup, with practical discoveries in things like metals, building materials, and weaponry. A less practical pursuit (to our eyes) was alchemy, where searches for how to transmute metals and brew elixirs of life went on for centuries with no success. Along the way, though, the alchemists learned how to distill, purify, and classify the substances they worked with, and they laid the foundations for modern chemistry without realizing it. Sometime in the 1600s, in the twilight of alchemy, the sun began to come up on what we would recognize as modern science. Discovery built on discovery as the new breed of natural scientists learned how to do systematic, reproducible experiments. The 1700s eclipse everything before them, but the 1800s easily outdo them in turn.
The entries in this book dont have to be read in order, but heres a brief tour of what youll encounter if you do. Experiments with gases of all sorts were cutting-edge science in the 1700s and early 1800s, and studying them proved an ideal way to learn how elements combined into compounds. Electricity then provided a way to make new chemical reactions happen that had never been seen before, and the field struggled to make sense of all the new elements and transformations that were being found so quickly. Organic chemists were busy isolating new substances from plants and other natural sources, and attempts to understand their structures gradually led to the realization that chemical compounds formed complex three-dimensional shapes.
The nineteenth century was also the era when some of the simplest questions finally began to be answered: Why are some chemicals so brightly colored, while others are clear? Why are some of them silvery metals that can only be melted in the hottest furnaces, while others are gases, some lighter than air? What makes some of them give off light, or even burst into flames, if opened to the air? Before the 1800s, these questions must have seemed nearly impossible to reconcile into theories that made sense, but a huge amount of work and several key advances began to make that possible.
By the early twentieth century, it was becoming clear that many substances were polymersstartlingly long chains of simpler molecules joined end to end. Living cells themselves use several of these, and polymer chemists found themselves creating everything from rubber and cornstarch to polyethylene in a quest to understand how these compounds worked. Meanwhile, organic chemists and inorganic chemists found themselves unexpectedly joining forces to produce a huge array of organometallic compoundsnever before seenand analytical chemistry moved into territories that no one had even realized were possible, with techniques like mass spectrometry revealing the weights of individual types of molecules.
World War II had an extraordinary effect on all technological fields. The war began with biplanes but finished with jet engines and guided missiles, and chemistry underwent similar changes. Petroleum chemistry, radioisotopes, and antibiotics were just three fields that advanced almost beyond recognition, but all parts of the science sped up dramatically. Then, by the end of the 1950s, DNA and protein sequences were recognized as being the keys to living systems and were deciphered for the first time during the 1960s. Analytical chemists were changing the science with new kinds of chromatography and NMR (nuclear magnetic resonance) machines, and medicinal chemists were taking the compounds of nature (antibiotics and steroids) and modifying their very structures.
The 1970s and 1980s saw the beginnings of molecular biology, a field that has moved biologists ever closer to chemistrys point of view. Chromatography and mass spectrometry began to merge into the most powerful analytical techniques ever developed, and the revolution in computer processing power turned X-ray crystallography calculations into just another afternoon in the lab.
The last twenty years have seen the rise of nanotechnology, with chemists starting to design and build molecular tools and scaffolds that would have been impossible to figure out in an earlier era. There has been a similar explosion of effort in whats now called chemical biology, using the techniques of chemistry to alter, probe, and understand proteins and other molecules of life. New organic chemistry reactions, new analytical equipment, and new computational power have all come together to make the field what it is today. If were going to take carbon dioxide out of the air and turn it back into useful compounds and fuels in order to keep new pollutants from even being used while cleaning up the old ones, if were going to synthesize new medicines or make new exotic materials stronger and lighter than anything before, well need these latest breakthroughs in chemistry.