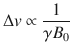1. NMR of Macromolecular Assemblies and Machines at 1 GHz and Beyond: New Transformative Opportunities for Molecular Structural Biology
Abstract
As a result of profound gains in sensitivity and resolution afforded by ultrahigh magnetic fields, transformative applications in the fields of structural biology and materials science are being realized. The development of dual low temperature superconducting (LTS)/high-temperature superconducting (HTS) magnets has enabled the achievement of magnetic fields above 1 GHz (23.5 T), which will open doors to an unprecedented new range of applications. In this contribution, we discuss the promise of ultrahigh field magnetic resonance. We highlight several methodological developments pertinent at high-magnetic fields including measurement of 1H-1H distances and 1H chemical shift anisotropy in the solid state as well as studies of quadrupolar nuclei such as 17O. Higher magnetic fields have advanced heteronuclear detection in solution NMR, valuable for applications including metabolomics and disordered proteins, as well as expanded use of proton detection in the solid state in conjunction with ultrafast magic angle spinning. We also present several recent applications to structural studies of the AP205 bacteriophage, the M2 channel from Influenza A, and biomaterials such as human bone. Gains in sensitivity and resolution from increased field strengths will enable advanced applications of NMR spectroscopy including in vivo studies of whole cells and intact virions.
Perspective
In the past two decades, the field of magnetic resonance has realized astounding advancements. One critical achievement toward progress in the fields of structural biology, materials science, and clinical imaging (to name just a few) has been the development of higher magnetic field strengths []. Higher magnetic fields yield profound improvements in both sensitivity and resolution.
The greatest drawback of nuclear magnetic resonance (NMR) relative to other analytical methods is relatively low sensitivity. Increasing magnetic field strength is a compelling way to increase sensitivity, as the signal-to-noise ratio (SNR, i.e., sensitivity) scales approximately as the power of 3/2 with the magnetic field strength [].
Increased magnetic fields also yield improvements in resolution:
where is the linewidth. As with improvements in sensitivity, increased resolution allows for the study of more complex systems and visualization of finer structural details.
Beyond significant gains in sensitivity and resolution, increased magnetic field strengths are particularly powerful for the study of quadrupolar nuclei ( I > ). Quadrupolar nuclei yield very broad spectra (on the order of MHz) due to the strong coupling (expressed by the coupling constant, C Q) between the nuclear quadrupolar moment and the electric field gradient (EFG). At higher magnetic field strengths, the contributions of the second-order quadrupolar interaction are reduced, leading to narrower spectra [].
Both NMR and clinical magnetic resonance imaging (MRI) benefit from increased magnetic field strengths. (For reviews of applications of high-magnetic fields to MRI, see refs..) Here, we focus on advancements in technology and methodology as applied in the burgeoning field of NMR at field strengths above 1 GHz. We also discuss several recent applications of high-magnetic fields to the study of biological systems.
1.1 Current State of the Art
Numerous technological gains have promoted the advancement of NMR to current levels, where field strengths above 1 GHz (23.5 T) have recently been achieved (Fig. ].
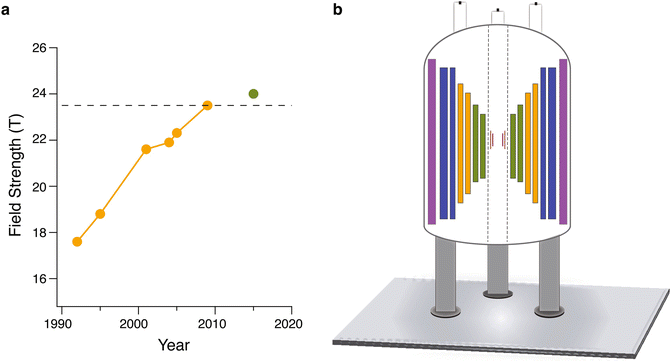
Fig. 1
( a ) Advancements in magnetic field strengths for NMR over the last few decades. Yellow indicates LTS magnets while green indicates dual LTS/HTS magnets. The dashed line indicates a frequency of 1 GHz (23.5 T). ( b ) General schematic of dual LTS/HTS magnet including shim coils and superconducting magnet coils. The precise number of LTS and HTS coils may vary. Purple represents superconducting shim coils, while blue represents outer NbTi LTS coils, orange represents Nb3Sn inner LTS coils, and green represents HTS coils. Within the magnet bore, maroon (outer) and pink (inner) bars represent the ferromagnetic and room temperature shim coils respectively
To date, work on ultrahigh field LTS/HTS magnets for biomolecular NMR applications has focused on proof of concept, with the bulk of published work done on the 24 T magnet at the National Institute for Materials Science (NIMS) developed in collaboration with RIKEN, Kobe Steel, and JEOL (Japan) [].
Beyond superconducting coil technology, for successful operation of ultrahigh field spectrometers advancements have needed to be made with respect to other spectrometer components, concurrent with the development of LTS/HTS magnets. Magnetic field stability and homogeneity are critical to successful experiments. A significant issue in the use of HTS technology for NMR is the inhomogeneous magnetic fields generated by the HTS materials. To compensate for these inhomogeneities in the LTS/HTS systems requires the use of ferromagnetic shim coils in addition to the standard superconducting and room temperature shims used in LTS magnets [].
The next stage in the evolution of increased magnetic field strengths for applications in NMR may be hybrid magnets, where an inner resistive coil is surrounded by an outer superconducting coil. NMR spectrometers currently in use utilize only superconducting coils, with 27 T the highest field achieved to date (the instrument setup for this system is not compatible with biomolecular NMR measurements due to the narrow bore size [] and Nb3Sn superconducting coils. Designing the resistive and superconducting coils to be powered in series rather than in parallel was a significant achievement that greatly reduced the size, in stability, and operating costs of the 36 T magnet relative to the 45 T hybrid magnet. An additional significant achievement of the 36 T magnet is the 40 mm bore size, large enough to accommodate the instrumentation needed for biomolecular NMR experiments. 1 GHz magnets currently produced by Bruker have a standard 54 mm bore. At present efforts are concentrated on achieving the high-field homogeneity needed for NMR experiments.
As mentioned above, increased magnetic field strengths in NMR will have a major impact in a significant number of fields including structural biology, materials science, and imaging. NMR studies of inorganic materials will greatly benefit from increased magnetic field strengths as many inorganic nuclei have large quadrupolar coupling constants and/or low natural abundance. Nuclei of particular interest for inorganic applications that have been shown to benefit from higher magnetic field strengths include 6Li ( I = 1, 7.6% natural abundance (NA) []). These elements are essential components of materials in many fields of inorganic chemistry research, including batteries and fuel cells, semiconductors, optical materials, metal organic frameworks (MOFs), catalysts, and glasses. NMR studies of these materials can yield valuable information often inaccessible by other methods including structure, oxidation state, hydrogen bonding environment, molecular motions, and effects of impurities.