1. A Synopsis of Proteins and Their Purification
Abstract
The isolation of a given protein, free of all other biomolecules, is the primary objective of any protein purification scheme. Classical chromatographic procedures have been designed to exploit particular distinguishing features of individual target proteins, such as size, physicochemical properties, and binding affinity. Advances in molecular biology and bioinformatics have positively contributed at every level to the challenge of purifying individual proteins and more recently have led to the development of high-throughput proteomic platforms. Here, a synopsis of developments in the field of protein chromatography is given, with reference to the principal tools and resources that are available to assist with protein purification processes.
The study of proteins and their associated functions is central to our understanding of virtually all fundamental biological processes. The term protein purification refers to a series of procedures that are designed to isolate a single protein type from a complex biological source such as tissue or a microbial culture. Proteins are probably the most commonly purified type of biological molecule as they are integral components of cellular structures and many biological processes, and among other roles are to be found as enzymes, scaffold molecules, cell signal transducers, and as components of gene regulatory complexes. A successful purification strategy is essential prior to performing structural and functional studies on a protein of interest. The various stages in the purification process may free the protein from a matrix in which it is confined, separate the protein from other nonprotein parts of the starting material, and finally separate the desired protein species from all other proteins present. The isolation of one protein, free of all other biomolecules, is the primary objective and separation procedures are designed to exploit any distinguishing features of the target protein, such as its size, its physicochemical properties, and its binding affinity.
Proteins consist of long chains of amino acids ( see Fig. ]. Some proteins are composed of more than one polypeptide, or more than one copy of the same polypeptide, that adapt a stable organized super-structural arrangement known as a quaternary structure. Following translation, the last step in the biosynthesis of many proteins involves posttranslational modification. Such modifications can include: (1) the addition of functional groups including carbohydrates (glycosylation), acetate (acylation), phosphates (phosphorylation), and lipids; (2) structural changes including proteolytic cleavage of the protein, disulfide bond formation between inter- or intra-chain cysteine residues, or racemization of proline residues; (3) covalent linkage to other polypeptides such as SUMO protein (SUMOylation), ubiquitin (ubiquitination), or ISG15 (ISGylation); and (4) chemical modification of individual amino acids by deamination, deamidation, or eliminylation. Proteins are therefore complex, fragile, and highly interconnected polymers.
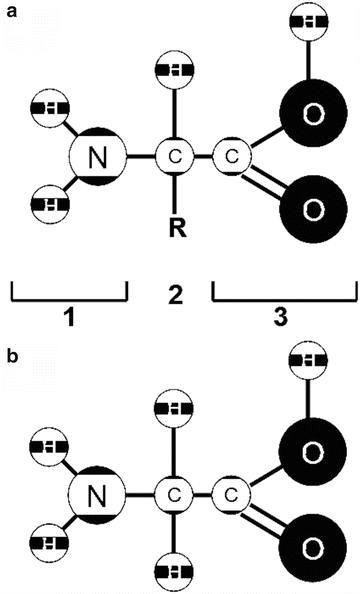
Fig. 1
Amino acid structure ( a ) with the amino group [] on the left , the amino acid side chain R [], and the carboxyl group [] on the right (the acidic group). The amino acid Glycine, shown in ( b ), with only a hydrogen atom as its side chain, is the smallest of the 20 amino acids
Table 1
Amino acid abbreviations and polarity
Amino acid name | Three-letter code | One-letter abbreviation | Polarity |
|---|
Alanine | Ala | A | Nonpolar, hydrophobic |
Arginine | Arg | R | Polar, charged, hydrophilic |
Asparagine | Asn | N | Polar, uncharged, hydrophilic |
Aspartic Acid | Asp | D | Polar, charged, hydrophilic |
Cysteine | Cys | C | Polar, uncharged, hydrophilic |
Glutamic Acid | Glu | E | Polar, charged, hydrophilic |
Glutamine | Gln | Q | Polar, uncharged, hydrophilic |
Glycine | Gly | G | Nonpolar, hydrophobic |
Histidine | His | H | Polar, charged, hydrophilic |
Isoleucine | Ile | I | Nonpolar, hydrophobic |
Leucine | Leu | L | Nonpolar, hydrophobic |
Lysine | Lys | K | Polar, charged, hydrophilic |
Methionine | Met | M | Nonpolar, hydrophobic |
Phenylalanine | Phe | F | Nonpolar, hydrophobic |
Proline | Pro | P | Nonpolar, hydrophobic |
Serine | Ser | S | Polar, uncharged, hydrophilic |
Threonine | Thr | T | Polar, uncharged, hydrophilic |
Tryptophan | Trp | W | Nonpolar, hydrophobic |
Tyrosine | Tyr | Y | Polar, uncharged, hydrophilic |
Valine | Val | V | Nonpolar, hydrophobic |
In order to determine the structure and function of any protein, it is first necessary to purify it. The history of protein purification dates back over 200 years to 1789 when Antoine Fourcroy reported on attempts to isolate substances from plants that had similar properties to egg albumen. One hundred years later, Franz Hofmeister obtained the first crystals of a protein, namely, ovalbumin, and now more than 50 years have elapsed since Max Perutz and John Kendrew used X-ray crystallography to decipher the first protein molecular structures, namely those of hamoglobin and myoglobin, and consequently receiving the Nobel prize in Chemistry [], and was still in recent use. Enzymes in particular became targets for purification and crystallization, with large-scale applications being developed in the food, detergent, and cosmetic industries among others. In many cases, these enzyme preparations, which were usually obtained from microbial sources and included amylases, lipases, and proteases, were pure enough in terms of activity as was necessary for their intended purpose. The purification processes involved were minimal, and such process enzymes were in fact not very pure at all. Most of the methodologies for purifying proteins from native sources were conceived in the 1960s and 1970s enabling small quantities of highly purified proteins to be obtained from large quantities of biological materials such as animal and plant tissues. Such were the quantities of materials needed that in one case, and by extrapolation, it would have necessitated starting with 500,000 sheep brains in order to produce 5 mg of sheep growth hormone. Since that time, the rapid progress made in molecular biology led to the point where theoretically any protein could be produced in recombinant form and in unlimited quantities from heterologous hosts such as bacteria, fungi, cell cultures of insect, plant and animal origin, and even whole animals and plants. In 1988, yeast-derived recombinant bovine chymosin, a new substitute for neo-natal calf rennet in cheese making, was the first recombinant enzyme to gain approval from regulatory authorities for use in food. The revolution in recombinant DNA technology brought additional improvements to almost every stage of recombinant protein production, and was accelerated even further with the introduction of the polymerase chain reaction (PCR) and associated methodologies. Rational approaches to Protein engineering by site-directed mutagenesis of the corresponding recombinant gene, gene fusion methods, gene codon content optimization, improvements to expression vectors, and tailoring of host cell genotypes all ensured that many problems with issues such as protein activity, stability, ease of purification, yields, posttranslational modifications, folding, and downstream processing could be addressed. Recombinant products such as protein hormones and their receptors, hamatology-associated proteins including clotting factors and clot-busting molecules, and proteins with immunomodulatory functions including antibodies and vaccine components are now big business. The Biopharma industry uses genetic information to discover, develop, manufacture, and commercialize recombinant bio-therapeutics that addresses significant medical needs.