Contents
Abbreviated Solutions to Problems
Fuller solutions to all chapter problems are published in the Absolute Ultimate Guide to Lehninger Principles of Biochemistry . For all numerical problems, answers are expressed with the correct number of significant figures.
Chapter 1
. (a) Diameter of magnified cell = 500 mm (b) 2.7 1012 actin molecules (c) 36,000 mitochondria (d) 3.9 1010 glucose molecules (e) 50 glucose molecules per hexokinase molecule
. ( a) 1 1012 g = 1 pg (b) 10% (c) 5%
. ( a) 1.6 mm; 800 times longer than the cell; DNA must be tightly coiled. (b) 4,000 proteins
. (a) Metabolic rate is limited by diffusion, which is limited by surface area. (b) 12 m1 for the bacterium; 0.04 m1 for the amoeba; surface-to-volume ratio 300 times higher in the bacterium.
. 2 106 s (about 23 days)
. The vitamin molecules from the two sources are identical; the body cannot distinguish the source; only associated impurities might vary with the source.
. (a)
(b)
(c)
(d)
(e)
(f)
.
The two enantiomers have different interactions with a chiral biological receptor (a protein).
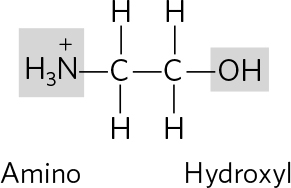
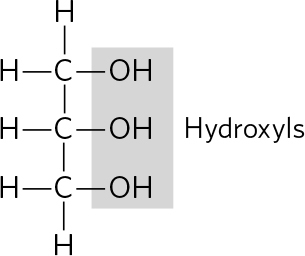
. (a) Only the amino acids have amino groups; separation could be based on the charge or binding affinity of these groups. Fatty acids are less soluble in water than amino acids, and the two types of molecules also differ in size and shapeeither of these property differences could be the basis for separation. (b) Glucose is a smaller molecule than a nucleotide; separation could be based on size. The nitrogenous base and/or the phosphate group also endow nucleotides with characteristics (solubility, charge) that could be used for separation from glucose.
. It is improbable that silicon could serve as the central organizing element for life, especially in an O2-containing atmosphere such as that of Earth. Long chains of silicon atoms are not readily synthesized; the polymeric macromolecules necessary for more complex functions would not readily form. Oxygen disrupts bonds between silicon atoms, and siliconoxygen bonds are extremely stable and difficult to break, preventing the breaking and making of bonds that is essential to life processes.
. Only one enantiomer of the drug was physiologically active. Dexedrine consisted of the single enantiomer; Benzedrine consisted of a racemic mixture.
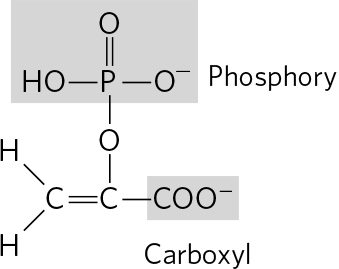
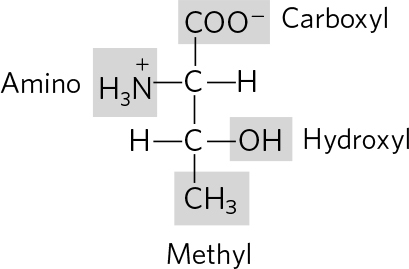
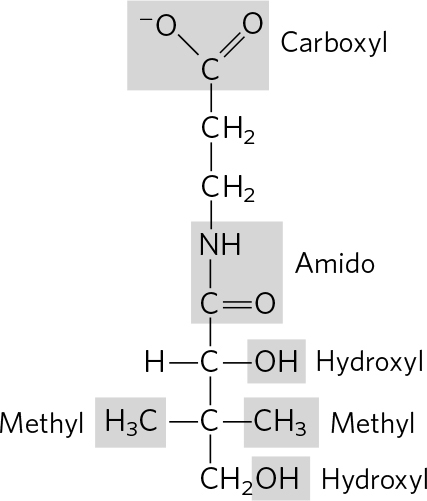
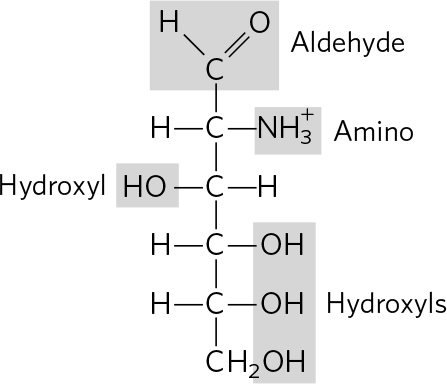
. (a) 3 Phosphoric acid groups; -D-ribose; guanine (b) Choline; phosphoric acid; glycerol; oleic acid; palmitic acid (c) Tyrosine; 2 glycines; phenylalanine; methionine
. ( a) CH2O; C3H6O3
(b)
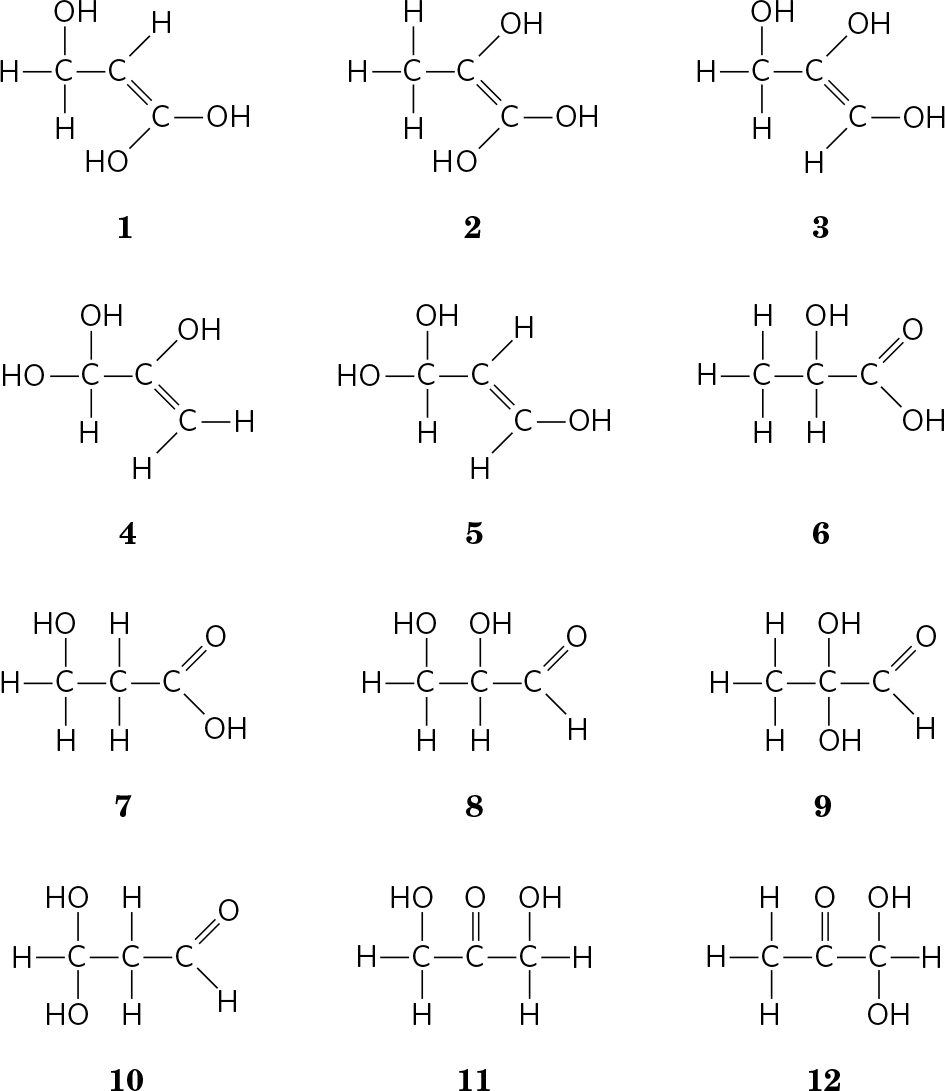
(c) X contains a chiral center; eliminates all but and . (d) X contains an acidic functional group; eliminates ; structure is consistent with all data. (e) Structure ; we cannot distinguish between the two possible enantiomers.
. The compound shown is ( R )-propranolol; the carbon bearing the hydroxyl group is the chiral carbon. ( S )-Propranolol has the structure:
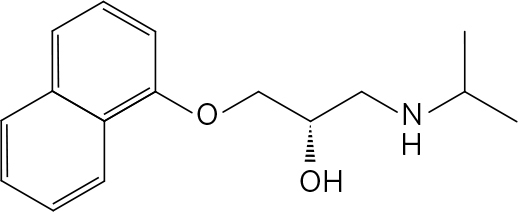
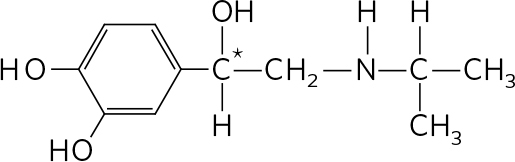
. The compound shown is ( S , S )-methylphenidate. ( R , R )-methylphenidate has the structure:
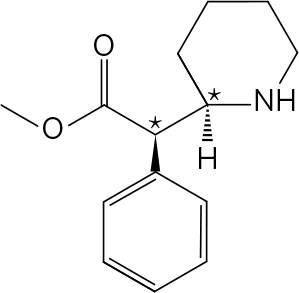
The chiral carbons are indicated with asterisks.
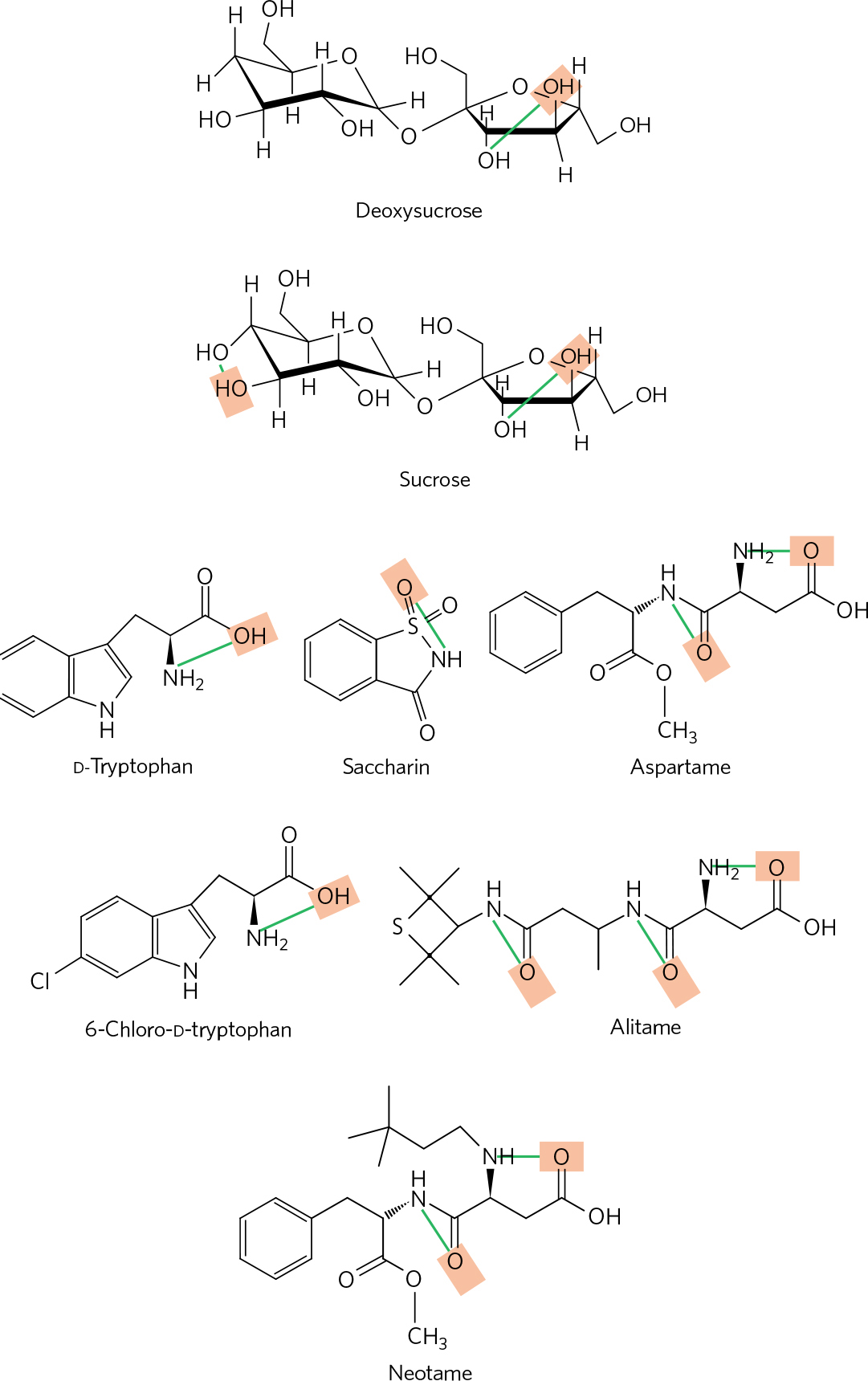
. (a) A more negative G corresponds to a larger K eq for the binding reaction, so the equilibrium is shifted more toward products and tighter bindingand thus greater sweetness and higher MRS. (b) Animal-based sweetness assays are time-consuming. A computer program to predict sweetness, eve n if not always completely accurate, would allow chemists to design effective sweeteners much faster. Candidate molecules could then be tested in the conventional assay. (c) The range 0.25 to 0.4 nm corresponds to about 1.5 to 2.5 single-bond lengths. The figure below can be used to construct an approximate ruler; any atoms in the light red rectangle are between 0.25 and 0.4 nm from the origin of the ruler.

There are many possible AH-B groups in the molecules; a few are shown here.



![David L Nelson - The Absolute, Ultimate Guide to Principles of Biochemistry [Study Guide and Solutions Manual]](/uploads/posts/book/288595/thumbs/david-l-nelson-the-absolute-ultimate-guide-to.jpg)














