1. Why Should I Read This Book?
Life on Earth has developed under constant exposure to radiation. In addition to ionising radiation from natural sources, a multitude of exposures from artificial sources produced by mankind came into play in the twentieth century. These radioactive sources were produced by some impressive developments in science and technology, and in turn they helped spur further developments, including of course in medical diagnostics and therapy .
Humans have no senses for ionising radiation. Perhaps this is because radiation from the natural environment does not present a hazard, and so there is no necessity for a warning. However, possible risks related to elevated levels of ionising radiation have often been underestimated. For example, it has happened several times that sources have been disposed of illegally, and later found by children. Since there is no apparent danger from these sources (no smell or taste is noticed, and nothing untoward can be seen) they are sometimes handled by the children and even stored in their homes. Considering the strength of radioactive sources used in medicine and technology, the irradiation from these sources over a period of several days can easily lead to radiation sickness and even death.
In the fifties and sixties of the last century, people were enthusiastic about nuclear energy. The nuclear weapon tests in the atmosphere in the early sixties led to high levels of radioactivity in the air and on the ground. Almost nobody was interested in this pollution at the time. One of the authors of this book (C.G.) was involved in the sixties in measurements of the radioactive pollution in the air at Kiel University. The level was so high that the local radio station was asked to include the radiation level (in pico-Curies per cubic metre) in the hourly weather forecast, and they did do so for about six months. It was stopped after that, because very few people were interested in the radiation levels. People born in the sixties were actually radiating babies , because even breast milk was contaminated with radioactive strontium (strontium-90) and caesium (caesium-137). This view about radiation pollution changed drastically with the Chernobyl disaster, after which considerable concern was raised, even where the contamination was at a very low level.
To make appropriate judgments about the potential danger caused by radioactive sources, it is important to develop a feeling for the biological effects of ionising radiation. It is impossible to eliminate radiation exposure altogether: one cannot possibly avoid natural radioactivity from the environment. Therefore, additional exposures have to be assessed with reference to natural levels of radiation exposure. To estimate the potential risk from radiation from the environment and from other sources, a minimum knowledge about physics, chemistry and biology is required: this can effectively serve as a sense for radioactivity , which we would otherwise be missing.
Fig. 1.1
Portrait of Henri Antoine Becquerel (drawing by C.G.)
Fig. 1.2
Portrait of Wilhelm Conrad Rntgen (drawing by C.G.)
Radioactivity was discovered by Henri Becquerel in 1896 (Figure radiation from the ore. However, the photosensitive paper was also blackened when the ore had not been previously exposed to light. The radiation spontaneously emerging from uranium was not visible to the human eye. Therefore, it was clear that he was dealing with a new phenomenon.
Shortly before this, in December 1895, Wilhelm Rntgen (Figure ) discovered X rays. This radiation emerged from materials when they were struck by energetic electrons. Indeed, the discovery by Rntgen had been one factor stimulating Becquerel to investigate fluorescence radiation from uranium ore.
The new research field of radioactivity became particularly important when, in 1898, Marie and Pierre Curie succeeded in isolating new radioactive elements (polonium and radium ) from uranium ore. Marie Curie was awarded two Nobel Prizes for her research (one in physics, one in chemistry).
At the turn of the century (18991902), investigations by Ernest Rutherford clearly demonstrated that there are different types of ionising radiation . Since initially it was impossible to identify these different types, they were named after the first three letters of the Greek alphabet:
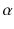
(alpha),
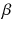
(beta) and
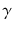
rays (gamma). It was shown that
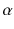
and
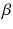
rays could be deflected by magnetic fields, and that
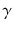
rays could not (see Figure ).
Fig. 1.3
Deflection of
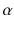
,
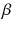
, and
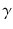
rays in a magnetic field. The magnetic field direction is downwards, into the page
This radioactivity was at first an unalterable phenomenon of the natural environment. Nobody was able to turn inactive materials into radioactive sources by chemical techniques. Nuclear physics technology, however, continued to develop, and in 1938, Otto Hahn and Fritz Stramann succeeded in inducing fission of uranium nuclei (although this discovery was accidental, as explained in Chapter ).
The importance of radioactivity and of radiation protection for mankind and the environment is substantial. The judgment on the effects of ionising radiation on humans should not be left only to so-called experts. Everybody who is prepared to get involved in these problems should be in a position to come to his own judgment. It is highly desirable that, for example, discussions on the benefits and risks of nuclear power and disposal of radioactive waste are not dominated by emotional antipathy or blind support, but rather by solid facts about radiation and radiation-related effects.












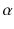 (alpha),
(alpha), 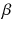 (beta) and
(beta) and 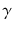 rays (gamma). It was shown that
rays (gamma). It was shown that 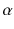 and
and 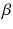 rays could be deflected by magnetic fields, and that
rays could be deflected by magnetic fields, and that 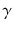 rays could not (see Figure ).
rays could not (see Figure ). 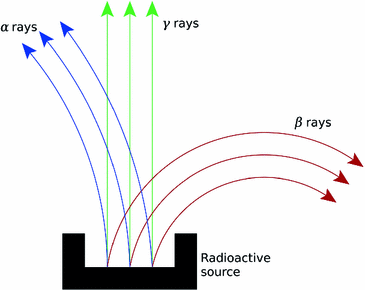
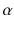 ,
, 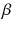 , and
, and 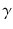 rays in a magnetic field. The magnetic field direction is downwards, into the page
rays in a magnetic field. The magnetic field direction is downwards, into the page