Chapter 1
Types of Radiation and Radioactive Decay
This chapter provides an overview of the types of radioactive decay and the three types of radiation: alpha, beta, and gamma rays.
Discovery and Initial Classification of Radiation
Henri Becquerel first discovered radioactivity in 1896 and shared the 1903 Nobel Prize in Physics with the Curies for his discovery. Before physicists understood the nature of the three types of radiation, Ernest Rutherford classified radiation into three types, alpha, beta, and gamma, according to how they are deflected in a magnetic field. Gamma rays are not affected by magnetic fields and therefore do not deflect. Alpha and beta radiation deflect in opposite directions.
Though physicists did not, at this stage, understand the different types of radiation, the behavior in magnetic fields provided important clues. Light and other forms of electromagnetic waves remain unaffected by magnetic fields. Positive and negative electric charges deflect in opposite directions in magnetic fields. Physicists discovered that gamma radiation is a very high energy electromagnetic wave and that alpha and beta radiation are positively and negatively charged particles.
Alpha Radiation
Alpha radiation consists of particles rather than rays. Alpha particles are helium nuclei, which contain two protons and two neutrons. Compared to most elementary particles, alpha particles have a relatively large mass and nuclear cross section. Nuclear physicists use the cross section to measure how likely a particle is to interact with matter.
The large cross section makes alpha radiation simultaneously both fairly safe and fairly lethal. Because alpha particles easily interact with matter, they are easily absorbed. These particles therefore have a short range and little penetrating power. Paper or ordinary indoor clothing can block most alpha particles. Hence it is quite easy to shield radioactive alpha sources to block the alpha particles.
If however one were to eat, drink, or breathe a source of alpha radiation, the emitted alpha particles would interact inside the body. A sufficiently large dose would be lethal despite the short range. Food or water contaminated with radioactive isotopes that undergo alpha decay can therefore be dangerous.
The decay of uranium into thorium provides a good example of a radioactive decay process emitting alpha radiation. Uranium-238 is an isotope of uranium with 92 protons and 146 neutrons in the nucleus. When uranium-238 undergoes alpha decay, it emits a helium nucleus leaving 90 protons and 144 neutrons in the nucleus. The nucleus thus decays from uranium into thorium. This decay is the first step in a decay series that continues through a series of alpha and beta decays until the original uranium-238 has decayed into lead-206.
For those unfamiliar with the terminology, isotopes are different forms of the same element in which the atoms have the same number of protons but different numbers of neutrons. All atoms of a particular element have the same number of protons, but the number of neutrons varies among different isotopes. The number in the isotope's name is the total number of protons and neutrons in the atomic nuclei of that isotope.
Beta Radiation
Like alpha radiation, beta radiation consists of particles rather than rays. Beta particles are either electrons or positrons, which are the antimatter particles corresponding to electrons.
Beta radiation has a little more range and penetrating power than alpha radiation. A sheet of aluminum at least a few millimeters thick is needed to block most beta particles. As for alpha radiation, beta radiation is most dangerous when one eats, drinks, or otherwise ingests a radioactive beta decay source.
In beta decay one of the neutrons in the atomic nucleus splits into a proton and an electron. The nucleus emits the electron, or beta particle, as well as an antineutrino produced in the reaction. The product (called daughter by physicists) nucleus has one more proton, and one less neutron, than the original nucleus. Hence it decays into a different element.
Neutrinos (and antineutrinos) are elusive nearly massless particles that carry off energy and momentum. They have such a small cross section that neutrinos almost never interact with matter. They just zip through the spaces between atoms, nuclei, and electrons interacting with nothing. Because they do not interact with matter neutrinos pose absolutely no radiation hazard.
In a similar process, called inverse beta decay, a proton emits a positron and a neutrino as it decays into a neutron. The daughter nucleus has one less proton and one more neutron than the original. A positron is the antimatter particle corresponding to an electron. Positrons have the same mass as electrons, but they have positive rather than negative electric charges. Physicists discovered positrons long after Rutherford originally classified the three types of radiation, so Rutherford did not observe their behavior in a magnetic field. As soon as they come into contact with electrons, positrons and electrons mutually annihilate each other, releasing gamma rays.
The decay of the radioactive isotope carbon-14, used in carbon dating, provides a good example of beta decay. A carbon-14 nucleus has 6 protons and 8 neutrons. After the beta decay it becomes nitrogen-14 with 7 protons and 7 neutrons. During the decay, the nucleus emits an electron and an antineutrino.
Gamma Radiation
Like light, radio waves, and X-rays, gamma rays are a type of electromagnetic wave. With the highest energy on the electromagnetic spectrum, gamma rays have much more penetrating power than either alpha or beta radiation and can pass through concrete walls. It takes several centimeters of lead to block gamma rays. Their high energy and penetrating power make gamma radiation dangerous.
Gamma decay occurs when a radioactive atomic nucleus is in a higher than normal energy level. The nucleus emits a gamma ray as it drops to its normal energy level.
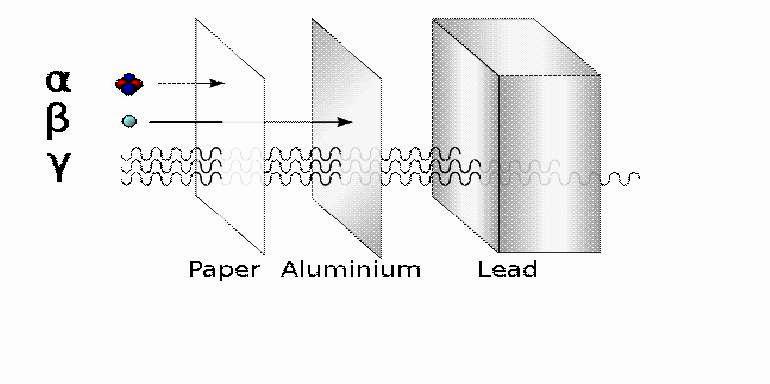
A Diagram Showing the Penetrating Abilities of Alpha, Beta, and Gamma Radiation
Image Credit: Wikimedia User Stannered/Creative Commons License
Electron Capture
Alpha, beta, and gamma decay are the most significant types of radioactive decay, but there is another type called electron capture. The nucleus of the atom captures one of the orbiting electrons. The electron then merges with one of the protons to form a neutron. The resulting nucleus has one less proton and one more neutron than the original nucleus. Think of electron capture as the opposite of beta decay. There is no need to worry about radiation from the electron capture process. Electron capture emits no radiation.
Chapter 2
Measuring Radiation and Radiation Dosages
This chapter describes the units physicists use to measure radiation dosages and ends by exploring common radiation dosages that are usually considered safe.












