Cover: Coloures-pic/Fotolia
Bibliographische Information der Deutschen Bibliothek
Die Deutsche Bibliothek verzeichnet diese Publikation in der Deutschen Nationalbibliographie; detaillierte bibliographische Daten sind im Internet ber http://dnb.ddb.de abrufbar.
Andreas Valet, Adalbert Braig
Light Stabilizers for Coatings
Hanover: Vincentz Network, 2017
European Coatings Library
ISBN 978-3-86630-132-0
2017 Vincentz Network GmbH & Co. KG, Hanover
Vincentz Network, Plathnerstr. 4c, 30175 Hanover, Germany
T +49 511 9910-033, F +49 511 9910-029,
This work is copyrighted, including the individual contributions and igures. Any usage outside the strict limits of copyright law without the consent of the publisher is prohibited and punishable by law. This especially pertains to reproduction, translation, micro ilming and the storage and processing in electronic systems.
Discover further books from European Coatings Library at:
www.european-coatings.com/shop
Layout: Danielsen Mediendesign, Hanover, Germany
eBook-Production:
readbox publishing, Dortmund, Germany
www.readbox.net
Foreword
Ah, happy he who still can hope to rise,
Emerging from this sea of fear and doubt!
What no man knows, alone could make us wise;
And what we know, we well could do without.
Goethe, Faust
Almost twenty years after the first edition of this book, the editor was asked by colleagues in the coating industry whether it would be possible to publish an up-dated second edition. When the editor asked us whether we would be interested in working on such a second edition, we agreed with great pleasure.
Although some time has passed since the first edition, the problems facing the industry are still the same: paint flaking off an object remains a serious concern. The object has lost its protection and is exposed to the elements. It has lost the colour that made it attractive. It has become insignificant and unnoticed.
The purpose of this book is to explain the underlying principles of paint degradation, demonstrate, with the help of numerous examples, how paint films can be protected and serve as a practical guide for formulators when selecting light stabilizers for their paint formulation.
For a more precise analysis of the mechanism of paint degradation and its prevention, the reader is referred to the extensive bibliography at the end of the book, which covers the subject comprehensively.
Hermann Hesse wrote Blue, yellow, white, red and green what wonderful colours []. When properly stabilized, colourful finishes ARE wonderful.
We would like to take this opportunity to thank our colleagues from the former Ciba Specialty Inc. and BASF Switzerland AG. Without their collaboration and support it would have never been possible to carry out the research, over a period of more than two decades, on which this book is based. We would also to thank Dr. Godwin Berner and Hans-Jrgen Berger who pioneered, built-up and led this successful business for so many years. Special thanks goes to Allan Cunningham for his great support editing our English text.
Basle, Switzerland June 2016
Andreas Valet and Adalbert Braig

Contents
Introduction
In the dictionary, paints are defined as liquid or powdered, solid substances which are applied thinly to objects and which dry by chemical reaction and/or physical changes to form a solid film whose function may be decorative and/or protective [].
Although applied only thinly, paints alter the appearance and increase the durability of many everyday objects. The efficiency of a paint, i.e. its ability to protect the coated object, is governed by the nature of the binder used in its formulation. Binders are also often referred to as film-forming agents, surface coating resins or synthetic resins. Paints contain organic solvents and/or water, or are completely solvent-free, depending on the kind of binder used. Paints may also contain pigments, fillers and other additives.
Paint films are exposed to all conditions arising in daily life, including mechanical stresses, chemicals and weathering, against which they must protect the coated object.
In 1860, A. Hofmann stated [] that the characteristic change which occurs in gutta-percha (rigid natural latex) when it has been in contact with air for some time, is well known. It becomes brittle and irreversibly loses its texture. Investigations had shown this change to be due to oxidation of the gutta-percha when exposed to air and Hofmanns statement is probably the first reference in the literature concerning the chemical reactions that alter polymer properties.
As a result, efforts began to protect polymers against these chemical reactions. New polymers required stabilizers to prevent them from deterioration in practical use. This formed the basis for the development of stabilizers for polymers. The term stabilizer refers to any additive that prevents or delays polymer degradation, irrespective of what kind of degradation mechanism is involved. The development of light stabilizers is described in the following publications and patent specifications.
Remarks on the Change of Gutta Percha under Tropical Influences []
Verfahren, um das Erhrten und Brchigwerden von Kautschuk, Guttapercha, Balata und hnlichen Gummiarten zu verhindern (Methods of preventing the hardening and embrittlement of rubber, gutta-percha, balata and similar rubbers) DP 221310; W. Ostwald, 1908)
The Chain Reaction Theory of Negative Catalysis[]
Autoxidation von Kohlenwasserstoffen: ber ein durch Autoxidation erhaltenes Tetrahydronaphthalin-Peroxid (Autoxidation of hydrocarbons: tetrahydronaphthalene peroxide obtained through autoxidation)[]
]
Pellicle and the Manufacture thereof (USP 2,129,131; E. Du Pont de Nemours, 1938
Vinylidene Chloride Composition Stable to Light (USP 2,264,291; The Dow Chemical Company, 1941)
Lichtschutzmittel und ihre Beurteilung (Light Stabilizers and their Assessment) []
Weather resistance of Cellulose Ester Plastics Compositions []
4-Benzoylresorcinol as an Ultraviolet Absorbent (USP 2,568,894; General Aniline & Film Corporation, 1951)
Verwendung von 2-Phenylbenzotriazol-Verbindungen zum Schtzen von organischem Material gegen ultraviolette Strahlung (Use of 2-phenylbenzotriazole compounds to protect organic materials from UV radiation) (DE 1185610; Ciba-Geigy AG, 1957)
Free Radical Reactions involving no Unpaired Electrons []
Stabilization of Synthetic Polymers (USP 3,542,729; Sankyo Ltd., 1970)
The preceding references formed the basis for the development of light stabilizers for coatings.
In this book, in addition to paint, two other terms are widely used in the industry will appear, namely coating and varnish.
Light and photo-oxidative degradation
2.1 Light
Light is generally described as radiation visible to the human eye comprising wavelengths between 400 and 750 nm[2]. But visible light is only a part of the electromagnetic radiation to which the earth is exposed. Electromagnetic radiation can be divided into different groups as shown in shows the sub-division of ultraviolet to infrared.
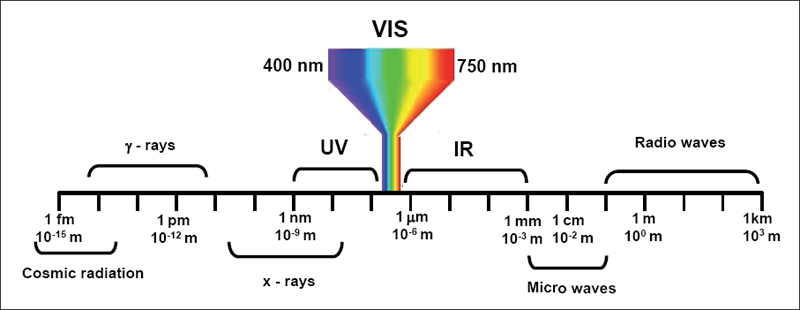
Figure 2.1: Classification of electromagnetic radiation with wavelengths of 10-15 to 103 m[]
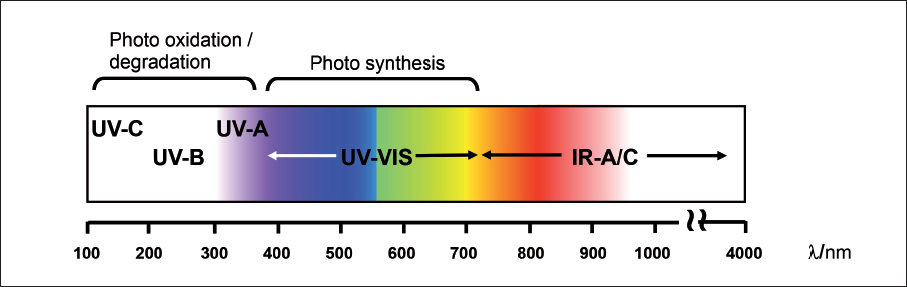
Next page














