Series Editor
Jack Legrand & Gilles Trystram
Handbook of Food Science and Technology 2
Food Process Engineering and Packaging
Romain Jeantet
Thomas Croguennec
Pierre Schuck
Grard Brul
First published 2016 in Great Britain and the United States by ISTE Ltd and John Wiley & Sons, Inc. Translated by Geraldine Brodkorb from Science des aliments Tec & Doc Lavoisier 2006.
Apart from any fair dealing for the purposes of research or private study, or criticism or review, as permitted under the Copyright, Designs and Patents Act 1988, this publication may only be reproduced, stored or transmitted, in any form or by any means, with the prior permission in writing of the publishers, or in the case of reprographic reproduction in accordance with the terms and licenses issued by the CLA. Enquiries concerning reproduction outside these terms should be sent to the publishers at the undermentioned address:
ISTE Ltd
27-37 St Georges Road
London SW19 4EU
UK
www.iste.co.uk
John Wiley & Sons, Inc.
111 River Street
Hoboken, NJ 07030
USA
www.wiley.com
ISTE Ltd 2016
The rights of Romain Jeantet, Thomas Croguennec, Pierre Schuck and Grard Brul to be identified as the authors of this work have been asserted by them in accordance with the Copyright, Designs and Patents Act 1988.
Library of Congress Control Number: 2016930387
British Library Cataloguing-in-Publication Data
A CIP record for this book is available from the British Library
ISBN 978-1-84821-933-5
Wiley End User License Agreement
Go to www.wiley.com/go/eula to access Wileys ebook EULA.
Introduction
Food is a complex and heterogeneous system. It often consists of a protein and/or polysaccharide matrix surrounding, sustaining a typically aqueous continuous phase containing soluble hydrophilic compounds (carbohydrates, salts, vitamins, etc.) and some dispersed elements (cells, fat globules, gas bubbles, crystals, etc.) (). Such a system is thermodynamically, biologically and chemically very unstable.
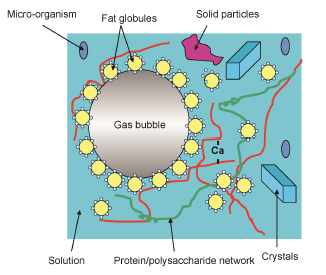
Matrix structure of food
The dispersed elements are subject to forces that cause phase separations either by sedimentation when the density of dispersed elements is greater than that of the dispersing phase, or by creaming when the opposite applies. Chemical potential or pressure gradients exerted on either side of the interfaces can induce the transfer of solutes and structural changes (coalescence, plasmolysis). This physicochemical instability can be increased by mechanical and thermal stresses on products during storage (refrigeration, freezing) and preparation (defrosting, reheating, etc.).
Protein and polysaccharide polymers that contribute significantly to the structuring of food are likely to reorganise during storage because of the influence of temperature on hydrophobic, ionic and hydrogen interactions. As described in Volume 1 [JEA 16a], recrystallization is sometimes accompanied by the release and migration of water with textural changes (starch retrogradation and bread staling).
Biological agents, enzymes and microorganisms find suitable conditions for their action and development in most foods both in terms of physicochemical conditions (pH, water activity aw and temperature) as well as availability of substrates and growth factors. Lipolysis, proteolysis and oxidation reactions, metabolite production (acid, alcohol, gas), the development of pathogenic flora and the release of toxins are all elements that negatively affect the sensory, safety and nutritional quality of food, as has already been demonstrated in the past. The same applies for chemical reactions (Maillard reaction, lipid autoxidation).
I.1. How to ensure biological stability of foods?
Four strategies can be used to ensure the biological stability of agricultural commodities and food ():
- inactivation of biological agents either through energy input, which induces a denaturation of enzymes and cell constituents, or through physical, chemical or enzymatic treatments (latter being able to lyse or alter some transport properties of the cell wall);
- separation of biological agents based on density differences or their size;
- inhibition of enzymes, microorganisms and reactions by decreasing water availability (lowering aw), which limits both the transfer of substrates and growth factors as well as the transfer of metabolites or reaction products that accumulate in the reaction environment;
- Inhibition through the creation of limiting physicochemical conditions (pH, oxygen pressure, bacterial inhibitors, etc.).
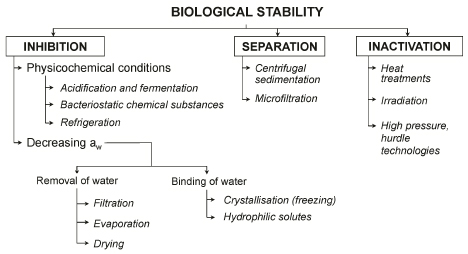
. Strategies for the biological stability of food
All these strategies will be detailed in of this book.
I.1.1. Thermal inactivation of biological agents
The destruction of microorganisms and the inactivation of enzymes by heat treatments that provide energy for the denaturation of macromolecules (proteins, DNA) were first applied by Appert and Pasteur. Food pasteurization and sterilization have been defined taking into account the thermal sensitivity of pathogenic flora:
- pasteurization destroys a large proportion of undesirable flora (Listeria, Staphylococci, Salmonella sp., etc.) and extends shelf life in the cold chain;
- sterilization destroys all microorganisms and denatures almost all enzymes (depending on the heat treatment) to allow long-term storage at room temperature.
To reduce the risk of pathogen proliferation, treatments are based on the thermal resistance of Clostridium botulinum, one of the most heat stable and pathogenic microorganisms. By aiming to destroy microorganisms and inactivate enzymes through thermal denaturation, macromolecules responsible for food texture and biological constituents (vitamins) are also partially or totally denatured. These side effects degrade the sensory and nutritional quality of food. Thus, successfully destroying microorganisms without reducing the food quality too much requires the precise control of heat transfers and momentum during heat treatments. Heat treatment is not always used due to the thermal sensitivity of some products (e.g. egg white) or in order to preserve the raw nature of the product. Other non-thermal treatments (i.e. inducing a temperature increase of less than 25C) are therefore used, for example ionization, high pressure and pulsed electric fields. These techniques have limited applications mainly due to cost, legislation and consumer acceptability. Hurdle technologies (combination of treatments to minimise energy input in the food product) may be an alternative.
I.1.2. Inhibition by decreasing water activity
Most microbial and biochemical changes that reduce the quality of food occur in the aqueous phase. Water has a dual role:
- as a solvent, it ensures the transfer of substrates, growth factors, biological agents and reaction products thereby creating optimum conditions for reactions;
- as a reaction substrate, it is involved in hydrolysis reactions (proteolysis, lipolysis).
Next page