
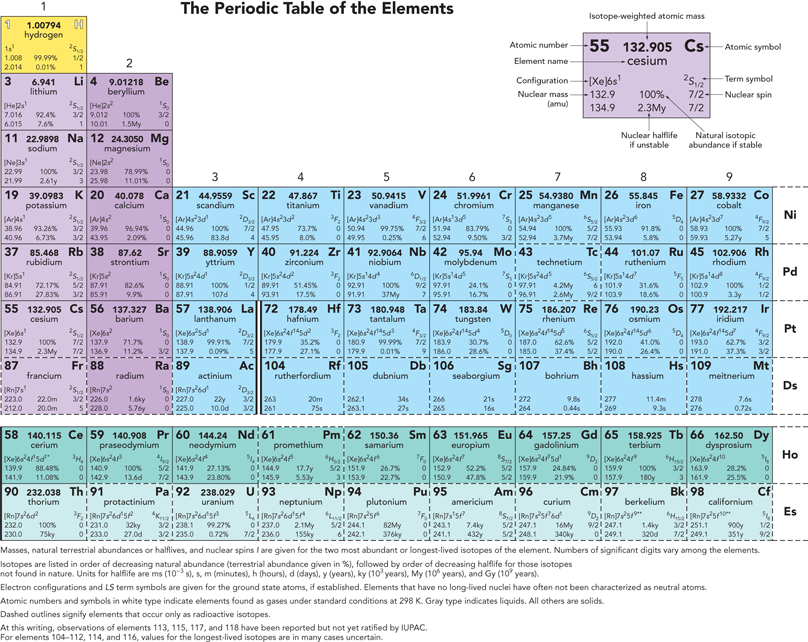
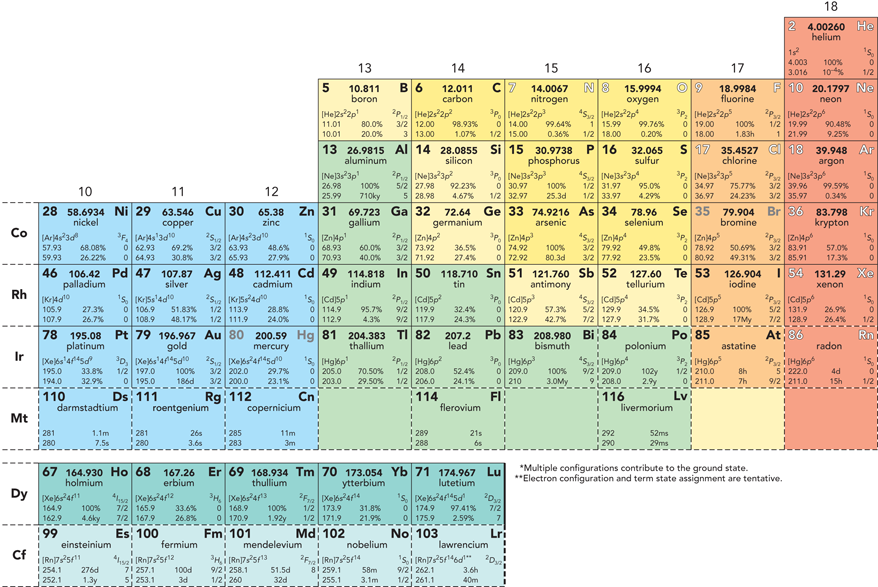
PHYSICAL CHEMISTRY Quantum Chemistry
and Molecular Interactions
PHYSICAL CHEMISTRY Quantum Chemistry
and Molecular Interactions
Andrew Cooksy

Boston Columbus Indianapolis New York San Francisco Upper Saddle River Amsterdam Cape Town Dubai London Madrid Milan Munich Paris Montral Toronto Delhi Mexico City So Paulo Sydney Hong Kong Seoul Singapore Taipei Tokyo
Editor in Chief: Adam Jaworski
Executive Editor: Jeanne Zalesky
Senior Marketing Manager: Jonathan Cottrell
Project Editor: Jessica Moro
Editorial Assistant: Lisa Tarabokjia
Marketing Assistant: Nicola Houston
Director of Development: Jennifer Hart
Development Editor: Daniel Schiller
Media Producer: Erin Fleming
Managing Editor, Chemistry and Geosciences: Gina M. Cheselka
Full-Service Project Management/Composition: GEX Publishing Services
Illustrations: Precision Graphics
Image Lead: Maya Melenchuk
Photo Researcher: Stephanie Ramsay
Text Permissions Manager: Joseph Croscup
Text Permissions Research: GEX Publishing Services
Design Manager: Mark Ong
Interior Design: Jerilyn Bockorick, Nesbitt Graphics
Cover Design: Richard Leeds, BigWig Design
Operations Specialist: Jeffrey Sargent
Cover Image Credit: Tony Jackson/Getty Images
Credits and acknowledgments borrowed from other sources and reproduced, with permission, in this textbook appear on the appropriate page within the text or in the back matter.
Copyright 2014 Pearson Education, Inc. All rights reserved. Manufactured in the United States of America. This publication is protected by Copyright, and permission should be obtained from the publisher prior to any prohibited reproduction, storage in a retrieval system, or transmission in any form or by any means: electronic, mechanical, photocopying, recording, or likewise. To obtain permission to use material from this work, please submit a written request to Pearson Education, Inc., Permissions Department, 1 Lake Street, Department 1G, Upper Saddle River, NJ 07458.
Many of the designations used by manufacturers and sellers to distinguish their products are claimed as trademarks. Where those designations appear in this book, and the publisher was aware of a trademark claim, the designations have been printed in initial caps or all caps.
Library of Congress Cataloging-in-Publication Data
Cooksy, Andrew.
Physical chemistry : quantum chemistry and molecular interactions / Andrew Cooksy. pages cm
Includes index.
ISBN-13: 978-0-321-81416-6
ISBN-10: 0-321-81416-9
1. Chemistry, Physical and theoretical--Textbooks. 2. Quantum chemistry--Textbooks. 3. Molecular dynamics--Textbooks. I. Title.
QD453.3.C655 2014
641--dc23
2012037314
1 2 3 4 5 6 7 8 9 10RRD16 15 14 13 12
ISBN-10: 0-321-81416-9
ISBN-13: 978-0-321-81416-6
 www.pearsonhighered.com
www.pearsonhighered.com
Dedication
To Mary, Wesley, and Owen
our great creative Mother, while she amuses us with apparently working in the broadest sunshine, is yet severely careful to keep her own secrets, and, in spite of her pretended openness, shows us nothing but results.
Nathaniel Hawthorne (18041864) The Birthmark
PHYSICAL CHEMISTRY BRIEF CONTENTS Quantum Chemistry and Molecular Interactions
BRIEF TABLE OF CONTENTS FOR COOKSyS THERMODYNAMICS, STATISTICAL MECHANICS, & KINETICS Thermodynamics, Statistical Mechanics, & Kinetics

A Introduction: Tools from Math and Physics
Classical Physical Chemistry Sets the Stage
Introduction to Statistical Mechanics: Building Up to the Bulk
Partitioning the Energy
Statistical Mechanics and Molecular Interactions
Mass Transport: Collisions and Diffusion
Energy Transport: Radiation and Matter
Introduction to Thermodynamics: Heat Capacity
The First Law: Expansion and Engines
The Second and Third Laws: Entropy
Phase Transitions and Phase Equilibrium
Solutions
The Thermodynamics of Chemical Reactions
Chemical Kinetics: Elementary Reactions
Chemical Kinetics: Multi-Step Reactions
DETAILED CONTENTS Physical Chemistry Quantum Chemistry and Molecular Interactions
To the Reader
This book is intended to provide students with a detailed guide to the reasoning that forms the basis for physical chemistrythe framework that unites all chemistry. The study of physical chemistry gives us the opportunity to look at our science as an integrated whole, with each concept connected to the next. My goal has been to trace those connections, step-by-step whenever possible, to show how each new concept makes sense given its place in the framework.
Because its ideas build upon each other in this way, physical chemistry can serve as the foundation for an intuitive understanding of chemistry in all its forms, whether synthesizing new compounds, analyzing samples in a forensic laboratory, or studying the properties of novel materials. To that end, this book emphasizes the shared, fundamental principles of chemistry, showing how we can justify the form and behavior of complex chemical systems by applying the laws of mathematics and physics to the structures of individual particles and then extrapolating to larger systems. We learn physical chemistry so that we can recognize these fundamental principles when we run into them in our other courses and in our careers. The relevance of this discipline extends beyond chemistry to engineering, physics, biology, and medicine: any field in which the molecular structure of matter is important.
A key step toward cultivating an intuition about chemistry is a thorough and convincing presentation of these fundamentals. When we see not only what the ideas are, but also how they link together, those ideas become more discernible when we examine a new chemical system or process. The following features of this text seek to achieve that objective.
My aim is to provide a rigorous treatment of the subject in a relaxed style. A combination of qualitative summaries and annotated, step-by-step derivations illuminates the logic connecting the theory to the parameters that we can measure by experiment. Although we use a lot of math to justify the theory we are developing, the math will always make sense if we look at it carefully. We take advantage of this to strengthen our confidence in the results and our understanding of how the math relates to the physics. Nothing is more empowering in physical chemistry than finding that you can successfully predict a phenomenon using both mathematics and a qualitative physical argument. The manifestation of atomic and molecular structure in bulk properties of materials is a theme that informs the unhurried narrative throughout the text.
Next page

















