1.1 Introduction
Moisturization of the skin is important for both cosmetic and medical purposes. The majority of moisturizing creams on the market are regulated as cosmetics; however, they may also be classified as pharmaceuticals (equivalent to medicinal products) or as a medical device within the European Member States. When they are regulated as pharmaceuticals or medical devices, they can also be marketed for treatment or prevention of diseases, such as atopic eczema, psoriasis, ichthyosis, and other hyperkeratotic skin diseases. There has been an increase in topically applied semi-solid formulations certified as medical devices used for the treatment of skin diseases in recent years [].
Moisturizing creams contain a number of different ingredients which can affect their suitability for the different types of dry skin conditions. In addition, these products come into close contact with our body, and they are generally used for long periods of time, which makes their compatibility with our body important.
The regulatory requirements and the approval processes for the three product categories are not the same; for example, cosmetics, and class I medical devices are not approved by any (outside) external/third party organization or authority before being placed on the market. On the other hand, medical devices class IIa, IIb, and III need third party (Notified Body) verification of fulfilling medical device regulation while pharmaceuticals go through rigorous evaluation and are granted a marketing authorization.
The present chapter will give an overview of the similarities and differences between the different regulatory categories. The borders will be described to help the reader understand the gray areas in classification between the categories.
1.2 Regulations and Classifications
Cosmetic products are currently regulated under the Cosmetic Directive 76/768/EEC and its amendments [].
Medicinal Products for Human Use (pharmaceuticals) is regulated under Directive 2001/83/EC and its amendments [].
Medical devices are regulated under the Medical Device Directive 93/42/EEC and its amendments [].
Other regulations may also be applicable, such as Regulation (EC) 1272/2008, classification, labeling and packaging of substances and mixtures, [] and the General Product Safety Directive 2001/95/EC.
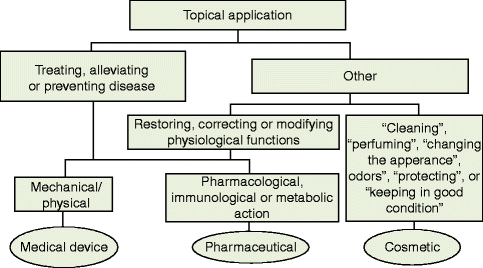
The borderlines between the different product categories may be difficult to demarcate. The intended use, mode of action, composition, physiological properties, and the risks of use are the bases to determine which set of regulations should be applied to a topical formulation, Fig. ].
Fig. 1.1
A schematic view on the classification of a medical device, a pharmaceutical and a cosmetic product
The definition of a cosmetic product is based on target of the application and the intended function []:
Any substance or mixture intended to be placed in contact with the various external parts of the human body (epidermis, hair system, nails, lips and external genital organs) or with the teeth and the mucous membranes of the oral cavity with a view exclusively or mainly to cleaning them, perfuming them, changing their appearance and or/correcting body odours and/or protecting them or keeping them in good condition.
The definition is thus based on two cumulative aspects, i.e. the target site of application and the intended main (cosmetic) function.
A medicinal product is defined either by virtue of its presentation or its function []. A product constitutes a medicinal product if it falls within either of these two categories:
Any substance or combination of substances presented as having properties for treating or preventing disease in human beings; or
Any substance or combination of substances which may be used in or administered to human beings either with a view to restoring, correcting or modifying physiological functions by exerting a pharmacological, immunological or metabolic action, or to making a medical diagnosis.
The terms for the actions are defined according to the following []:
Pharmacological action : interaction between the molecules of the substance in question and a cellular constituent, usually referred to as a receptor, which either results in a direct response, or which blocks the response to another agent. Although not a completely reliable criterion, the presence of a dose-response correlation is indicative of a pharmacological effect.
Immunological action : action in or on the body by stimulation and/or mobilisation of cells and/or products involved in a specific immune reaction.
Metabolic action : action which involves an alteration, including stopping, starting or changing the speed of the normal chemical processes participating in, and available for, normal body function. The fact that a product is metabolised by the human body does not necessarily mean that the substance contained in the product has a metabolic action upon the body.
A Medical Device is Defined in Article 1(2) of the Medical Device Directive as:
Any instrument, apparatus, appliance, software, material or other article, whether used alone or in combination, including the software intended by its manufacturer to be used specifically for diagnostic and/or therapeutic purposes and necessary for its proper application, intended by the manufacturer to be used for human beings for the purpose of:
diagnosis, prevention, monitoring, treatment or alleviation of disease,
diagnosis, monitoring, treatment, alleviation of or compensation for an injury or handicap,
investigation, replacement or modification of the anatomy or of a physiological process,
control of conception,
and which does not achieve its principal intended action in or on the human body by pharmacological, immunological or metabolic means, but which may be assisted in its function by such means.