Slide 1.1
This slide places Chap. in Part I of the book: Introduction to Analytical Chemistry. Also, it shows the other two parts and annexes.
This is an introductory chapter intended to serve as a general approach to Analytical Chemistry.
Slide 1.2
1.2.1 . The nine sections of the chapter. After placing the chapter in the context of Part I, it provides a general description of Analytical Chemistry in the next four sections. Through conceptual and technical hierarchies and classifications, the contents of the discipline are established its essential key words identified.
1.2.2 . The teaching aims to be fulfilled are defined: essentially, to provide an overview of Analytical Chemistry as the third basic component of Chemistry through its landmarks.
1.1.1 Introduction to Part I (1 Slide)
Slide 1.3
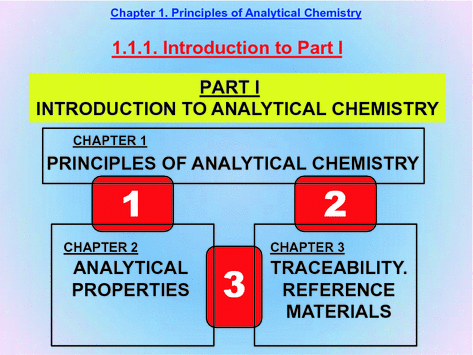
This is a schematic depiction of the relationships (boundaries 13) among the contents of the first three chapters, which together provide a general, harmonic overview of Analytical Chemistry.
Chapter introduces the general principles of Analytical Chemistry and is connected with the other two as follows:
Boundary 1. Analytical Chemistry uses a series of indicators to assess analytical quality (Chap..
Boundary 2. Traceability, both internal and external, is essential with a view to acquiring an accurate image of Analytical Chemistry, which is the discipline of (bio)chemical measurements: measuring requires comparing with standards (reference materials) and, inevitably, assuring traceability.
Boundary 3. This boundary relates Chaps.. The connection between quality-related analytical indicators and the analytical properties to be maximized (accuracy and representativeness) is closely related to the integral concept of traceability of analytical results (see Slide 3.25). Also, quality-related analytical indicators rely critically on the reference materials used for (bio)chemical measurements.
1.1.2 Definitions of Analytical Chemistry (4 Slides)
The following slides provide various supplementary definitions of Analytical Chemistry intended to construct an identity of its own as an essential discipline of Chemistry.
Slide 1.4
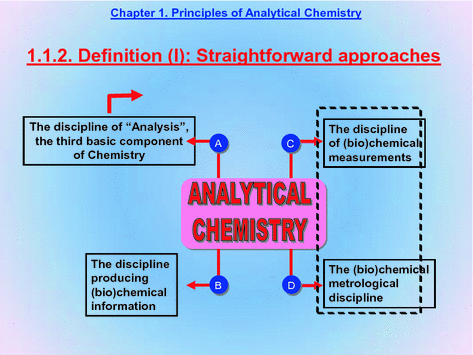
1.4.1 . This is a compilation of straightforward approaches to defining Analytical Chemistry.
First, Analytical Chemistry is defined as the discipline of (Bio)chemical Analysis and hence as the third essential element of Chemistry as shown in the next two slides.
1.4.2 . Analytical Chemistry is the discipline of Chemistry in charge of producing quality (bio)chemical information. This is the output of Analysis, the central element in the previous paragraph.
1.4.3 . Analytical Chemistry is thus the discipline of (bio)chemical measurements.
1.4.4 . And hence the (bio)chemical metrology discipline since Metrology is the science of measurements, whether physical (temperature), chemical (calcium concentration in milk), biochemical (enzyme activity in a biological fluid), microbiological (bacterial count in a culture) or otherwise.
As a result, the last two definitions are identical. In fact, they show where Metrology and Analytical Chemistry converge. As shown later on, however, their coincidences have synergistic connotations.
Slide 1.5
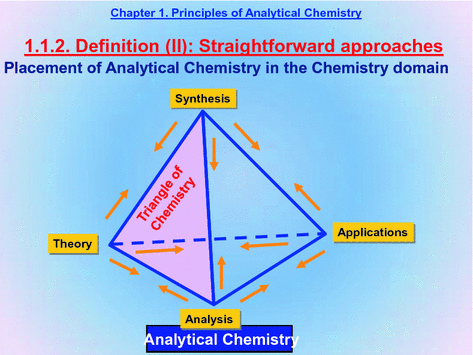
1.5.1 . This slide places Analysis (Analytical Chemistry) in the context of Chemistry as an essential ingredient of its definition.
Thus, Analysis is an apex of the basic triangle defining Chemistry in addition to Theory and Synthesis.
1.5.2 . Applications are also essential for Chemistry. As a result, so the basic triangle of Chemistry becomes a tetrahedron.
1.5.3 . The tetrahedron affords two- and three-way relationships among each component of Chemistry and those at the other apices. Thus, Synthesis provides the reagents needed for Analysis and Analysis is indispensable to characterize raw materials, intermediate products and end-products in a chemical synthesis process.
In addition, the tetrahedron distinguishes Analysis from Applications of Chemistry, which is essential in order to define Analytical Chemistry thoroughly.
1.5.4 . Analysis definitely falls in the domain of Analytical Chemistry.
Slide 1.6
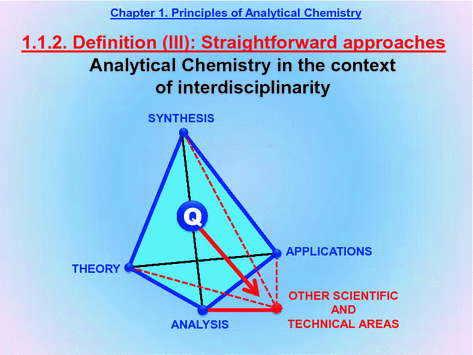
The tetrahedron in Slide 1.5 must be expanded to a pentahedron in order to accurately define Chemistry in the XXI century by adding another apex: boundaries to other scientific and technical areas.