Table of Contents
List of tables
- Tables in Chapter 1
- Tables in Chapter 2
- Tables in Chapter 4
- Tables in Chapter 6
List of illustrations
- Figures in Introduction
- Figures in Chapter 1
- Figures in Chapter 2
- Figures in Chapter 3
- Figures in Chapter 4
- Figures in Chapter 5
Landmarks
Table of Contents
Bone Remodeling Process
Mechanics, Biology, and Numerical Modeling
Rabeb Ben Kahla
Laboratoire de Systmes et de Mcanique Applique (LASMAP), Ecole Polytechnique de Tunis Universit De Carthage, La Marsa, Tunisie
Laboratoire de Mcanique Applique et Ingnierie (LR-MAI), Ecole Nationale dIngnieurs de Tunis Universit Tunis El Manar, Tunis, Tunisie
Abdelwahed Barkaoui
Laboratoire des Energies Renouvelables et Matriaux Avancs (LERMA), Universit Internationale de Rabat, Rabat-Sala El Jadida, Morocco

Copyright
Academic Press is an imprint of Elsevier
125 London Wall, London EC2Y 5AS, United Kingdom
525 B Street, Suite 1650, San Diego, CA 92101, United States
50 Hampshire Street, 5th Floor, Cambridge, MA 02139, United States
The Boulevard, Langford Lane, Kidlington, Oxford OX5 1GB, United Kingdom
Copyright 2021 Elsevier Inc. All rights reserved.
No part of this publication may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopying, recording, or any information storage and retrieval system, without permission in writing from the publisher. Details on how to seek permission, further information about the Publishers permissions policies and our arrangements with organizations such as the Copyright Clearance Center and the Copyright Licensing Agency, can be found at our website: www.elsevier.com/permissions.
This book and the individual contributions contained in it are protected under copyright by the Publisher (other than as may be noted herein).
Notices
Knowledge and best practice in this field are constantly changing. As new research and experience broaden our understanding, changes in research methods, professional practices, or medical treatment may become necessary.
Practitioners and researchers must always rely on their own experience and knowledge in evaluating and using any information, methods, compounds, or experiments described herein. In using such information or methods they should be mindful of their own safety and the safety of others, including parties for whom they have a professional responsibility.
To the fullest extent of the law, neither the Publisher nor the authors, contributors, or editors, assume any liability for any injury and/or damage to persons or property as a matter of products liability, negligence or otherwise, or from any use or operation of any methods, products, instructions, or ideas contained in the material herein.
British Library Cataloguing-in-Publication Data
A catalogue record for this book is available from the British Library
Library of Congress Cataloging-in-Publication Data
A catalog record for this book is available from the Library of Congress
ISBN: 978-0-323-88467-9
For Information on all Academic Press publications visit our website at https://www.elsevier.com/books-and-journals
Publisher: Mara Conner
Acquisitions Editor: Fiona Geraghty
Editorial Project Manager: Leticia M. Lima
Production Project Manager: Sojan P. Pazhayattil
Cover Designer: Christian J. Bilbow
Typeset by MPS Limited, Chennai, India

Introduction
The skeletal system plays an essential support role for the entire human body. It supports the gravity forces and the stresses produced by daily activities. The bone thus optimizes and adapts its mass and geometry through the remodeling process. Mechanically, bone is a living, nanocomposite material with a complex hierarchical structure that gives bone remarkable mechanical properties: light weight, high rigidity, toughness, and fracture resistance. The imbalance in bone remodeling is responsible for certain bone pathologies such as osteoporosis and Pagets disease. In particular, osteoporosis induces a loss of bone mass as well as a reduction in the quality of bone tissue (microarchitecture). The architecture and the structural properties of the bone are thus degraded, which causes a decrease in bone quality and therefore, an increase of fractures risk.
Throughout life, bone is constantly remodeled through the complementary resorption and formation activities, establishing what is known as bone remodeling process (). This process requires a highly coordinated regulation in time and space to consistently maintain bone amount and quality. This coordination mainly incorporates bone-resorbing osteoclasts and bone-forming osteoblasts, which are the major two actors in the remodeling event. The delicate balance between the resorbed bone amount and the subsequent deposited amount requires a strict coordination of the resorption and the formation activities, allowing to generate the appropriate osteoblast number in remodeling area, which is referred to as the coupling mechanism. Moreover, the coordination between osteoblast and osteoclast activities involves other cells from diverse origins, in addition to several hormones, cytokines, and growth factors that tightly interlink osteoblast- and osteoclast-lineage cells through a complex interaction network throughout the remodeling cycle.
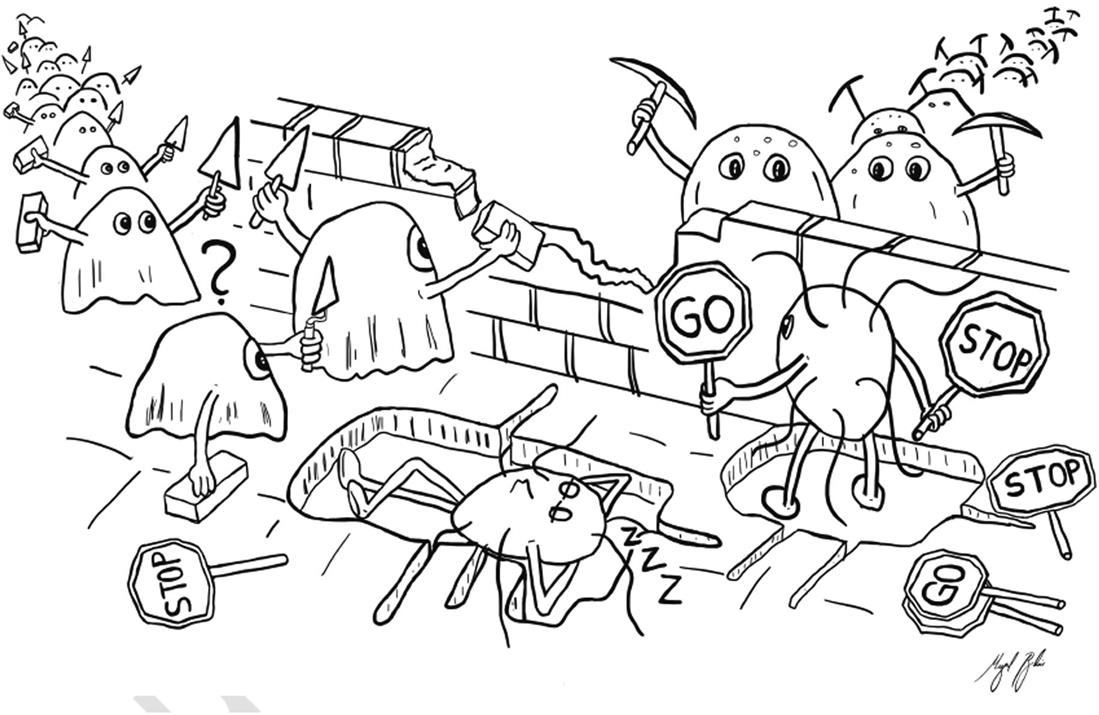
Figure 1 Cartoon representing the bone remodeling process with the osteoblast-mediated bone formation and the osteoclast-mediated bone resorption driven by osteocytes. Bahia, M. T., Hecke, M. B., Mercuri, E. G. F., & Pinheiro, M. M. (2020). A bone remodeling model governed by cellular micromechanics and physiologically based pharmacokinetics. Journal of the Mechanical Behavior of Biomedical Materials, 104, 103657.
The remodeling process is an integral part of the calcium homeostatic system and provides a crucial mechanism for old bone removal, as well as for bone damage repair and adaptation to physical stress, allowing to maintain the skeleton mechanical integrity. The remodeling process manifests at anatomically distinct sites known as bone multicellular units, each unit functioning asynchronously and independently from other units throughout the skeleton. It should however be noted that the bone multicellular units in cortical and in trabecular bones greatly differ in their structure, as well as in the way the bone is removed and replaced.
The concept of bone remodeling compartment consists of initiating the remodeling process within a canopy, and intercellular communication occurs in this compartment among the component bone cells, from vascular and endothelial cells, and probably from immune cells reaching the remodeling sites via the blood supply.
Bones occupy about 15% of the whole body weight, a fraction that does not deserve any consequence interpretation. Interestingly, human body ambulation, ventilation, and protection are primarily associated with bone, which highlights bone mechanical function. Therefore bone represents a structural material with mechanical characteristics resembling any other material with a mineral-based structure, even if the discovery process and the investigation of the relation between microcomponents and bulk material is opposite for the two types of material. After more than 2000 years of improvements, we now have enough knowledge of the right components to make a high quality steel, but still do not know yet how to efficiently treat bone metabolic disorders and age-related diseases, including osteoporosis. Steel is inert material, whereas bone is a living material. Steel is a mineral material, whereas bone is a biological material. Steel structure alters with higher mechanical loads, whereas bone strengthens with higher mechanical loads. All of these are differential features clearly show that bone mechanics do not necessarily follow the same classic rules of continuum damage mechanics as the remaining structures. This goes back to several poorly known factors and mechanisms, according to which bone structure is maintained.